Neuronal Differentiation from Induced Pluripotent Stem Cell-Derived Neurospheres by the Application of Oxidized Alginate-Gelatin-Laminin Hydrogels
- PMID: 33808044
- PMCID: PMC8000907
- DOI: 10.3390/biomedicines9030261
Neuronal Differentiation from Induced Pluripotent Stem Cell-Derived Neurospheres by the Application of Oxidized Alginate-Gelatin-Laminin Hydrogels
Abstract
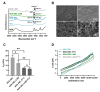
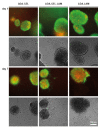
Biodegradable hydrogels that promote stem cell differentiation into neurons in three dimensions (3D) are highly desired in biomedical research to study drug neurotoxicity or to yield cell-containing biomaterials for neuronal tissue repair. Here, we demonstrate that oxidized alginate-gelatin-laminin (ADA-GEL-LAM) hydrogels facilitate neuronal differentiation and growth of embedded human induced pluripotent stem cell (hiPSC) derived neurospheres. ADA-GEL and ADA-GEL-LAM hydrogels exhibiting a stiffness close to ~5 kPa at initial cell culture conditions of 37 °C were prepared. Laminin supplemented ADA-GEL promoted an increase in neuronal differentiation in comparison to pristine ADA-GEL, with enhanced neuron migration from the neurospheres to the bulk 3D hydrogel matrix. The presence of laminin in ADA-GEL led to a more than two-fold increase in the number of neurospheres with migrated neurons. Our findings suggest that laminin addition to oxidized alginate-gelatin hydrogel matrices plays a crucial role to tailor oxidized alginate-gelatin hydrogels suitable for 3D neuronal cell culture applications.
Keywords: bioprinting; human induced pluripotent stem cells (hiPSC); hydrogels; laminin; neurospheres; oxidized alginate; tissue engineering.
Conflict of interest statement
Authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

