Molecular Chaperones in Osteosarcoma: Diagnosis and Therapeutic Issues
- PMID: 33808130
- PMCID: PMC8067202
- DOI: 10.3390/cells10040754
Molecular Chaperones in Osteosarcoma: Diagnosis and Therapeutic Issues
Abstract
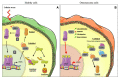
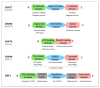
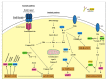
Osteosarcoma (OS) is the most common form of primary bone tumor affecting mainly children and young adults. Despite therapeutic progress, the 5-year survival rate is 70%, but it drops drastically to 30% for poor responders to therapies or for patients with metastases. Identifying new therapeutic targets is thus essential. Heat Shock Proteins (HSPs) are the main effectors of Heat Shock Response (HSR), the expression of which is induced by stressors. HSPs are a large family of proteins involved in the folding and maturation of other proteins in order to maintain proteostasis. HSP overexpression is observed in many cancers, including breast, prostate, colorectal, lung, and ovarian, as well as OS. In this article we reviewed the significant role played by HSPs in molecular mechanisms leading to OS development and progression. HSPs are directly involved in OS cell proliferation, apoptosis inhibition, migration, and drug resistance. We focused on HSP27, HSP60, HSP70 and HSP90 and summarized their potential clinical uses in OS as either biomarkers for diagnosis or therapeutic targets. Finally, based on different types of cancer, we consider the advantage of targeting heat shock factor 1 (HSF1), the major transcriptional regulator of HSPs in OS.
Keywords: HSF1; HSPs; bone tumor; osteosarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

