Real Life Use of Bendamustine in Elderly Patients with Lymphoid Neoplasia
- PMID: 33808164
- PMCID: PMC8066290
- DOI: 10.3390/jpm11040249
Real Life Use of Bendamustine in Elderly Patients with Lymphoid Neoplasia
Abstract
Background: Bendamustine is a cytotoxic alkylating drug with a broad range of indications as a single agent or in combination therapy in lymphoid neoplasia patients. However, its tolerability in elderly patients is still debated.
Methods: An observational, retrospective study was carried out; patients with chronic lymphocytic leukemia (CLL) or lymphoma, aged ≥ 65 years old, treated with bendamustine-based regimens in first or subsequent lines between 2010 and 2020 were considered eligible.
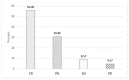
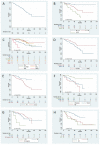
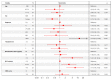
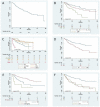
Results: Overall, 179 patients aged ≥ 65 years were enrolled, 53% between 71 and 79 years old. Cumulative Illness Rating Scale (CIRS) comorbidity score was ≥6 in 54% patients. Overall survival (OS) at 12 months was 95% (95% confidence interval [CI]: 90-97%); after a median follow up of 50 months, median OS was 84 months. The overall response rate was 87%, with 56% complete responses; the median time to progression (TTP) was 61 months. The baseline factors affecting OS by multivariable analysis were sex, histological diagnosis, renal function, and planned bendamustine dose, while only type of lymphoma and bendamustine dose impacted on TTP. Main adverse events were neutropenia (grade ≥ 3: 43%) and infections (any grade: 36%), with 17% of patients requiring hospital admission.
Conclusions: The responses to bendamustine, as well as survival, are relevant even in advanced age patients, with a manageable incidence of acute toxicity.
Keywords: bendamustine; chronic lymphocytic leukemia; efficacy; elderly; lymphoma; safety.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cheson B.D., Brugger W., Damaj G., Dreyling M., Kahl B., Kimby E., Ogura M., Weidmann E., Wendtner C.-M., Zinzani P.L. Optimal use of bendamustine in hematologic disorders: Treatment recommendations from an international consensus panel—An update. Leuk. Lymphoma. 2016;57:766–782. doi: 10.3109/10428194.2015.1099647. - DOI - PMC - PubMed
-
- Kater A.P., Wu J.Q., Kipps T., Eichhorst B., Hillmen P., D’Rozario J., Assouline S., Owen C., Robak T., de la Serna J., et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations From the MURANO Phase III Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:4042–4054. doi: 10.1200/JCO.20.00948. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

