Characterizing and Quantifying Arbovirus Transmission by Aedes aegypti Using Forced Salivation and Analysis of Bloodmeals
- PMID: 33808172
- PMCID: PMC8065531
- DOI: 10.3390/insects12040304
Characterizing and Quantifying Arbovirus Transmission by Aedes aegypti Using Forced Salivation and Analysis of Bloodmeals
Abstract
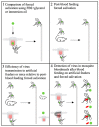
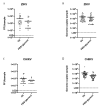
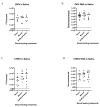
Arbovirus transmission studies are dependent on the ability to estimate the titer of virus transmitted from infectious mosquitoes to a host. There are several methods for estimating virus titer in mosquito saliva, including (1) using forced salivation (FS) whereby the infectious mosquito's proboscis is forced into a capillary tube containing media to collect and test their saliva for virus, and (2) by quantifying virus expectorated into host tissues or into the blood contained in an artificial feeder immediately after blood feeding. We studied FS and bloodmeals to estimate and compare titers of Zika virus and chikungunya virus transmitted by the mosquito vector Aedes aegypti. Infectious virus and viral genomes of both viruses were detected more often from individual mosquitoes using immersion oil for the FS media compared to fetal bovine serum (FBS) plus glycerol, but the FS media had no influence on virus quantification from positive samples. FS virus titers were equivalent when comparing individuals or groups of mosquitoes that never received a blood meal compared to those that were blood fed immediately prior, showing that blood feeding does not influence FS. This suggested that performing FS on mosquitoes after blood feeding might be an efficient way to estimate virus transmitted during blood feeding. However, detecting virus from the blood remaining in an artificial feeder post-blood feeding was mostly unsuccessful relative to quantifying virus from FS of the post-blood fed mosquitoes. In contrast, immunocompromised mice always became infected after being fed on by Zika-infected mosquitoes, even when no infectious virus was detected in their saliva by FS post-blood feed. Due to this discrepancy, we tested the ingested bloodmeals of individual mosquitoes that fed on artificial blood feeders for virus, and compared these to virus in their saliva harvested from FS and to virus in their bodies. These experiments revealed ~50-100 times higher virus titers in the dissected bloodmeals compared to those detected in the same mosquitoes' saliva, demonstrating how mosquitoes re-ingest much of their saliva during artificial blood feeding, and highlighting a large increase in virus transmission during Aedes aegypti blood feeding. Both FS and the dissected bloodmeals of artificially blood-fed mosquitoes showed that the quantity of viral RNA expectorated by mosquitoes was 2-5 logs more than the quantity of infectious virus. The results from this study add critical information to understanding and quantifying the transmission of Aedes aegypti arboviruses.
Keywords: Aedes aegypti; arboviruses; saliva; transmission.
Conflict of interest statement
The authors declare no conflict of interest
Figures

Similar articles
-
Saliva collection via capillary method may underestimate arboviral transmission by mosquitoes.Parasit Vectors. 2022 Mar 24;15(1):103. doi: 10.1186/s13071-022-05198-7. Parasit Vectors. 2022. PMID: 35331315 Free PMC article.
-
Differential outcomes of Zika virus infection in Aedes aegypti orally challenged with infectious blood meals and infectious protein meals.PLoS One. 2017 Aug 10;12(8):e0182386. doi: 10.1371/journal.pone.0182386. eCollection 2017. PLoS One. 2017. PMID: 28796799 Free PMC article.
-
The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti.PLoS Negl Trop Dis. 2016 Apr 28;10(4):e0004677. doi: 10.1371/journal.pntd.0004677. eCollection 2016 Apr. PLoS Negl Trop Dis. 2016. PMID: 27124663 Free PMC article.
-
Vector competence of populations of Aedes aegypti from three distinct cities in Kenya for chikungunya virus.PLoS Negl Trop Dis. 2017 Aug 18;11(8):e0005860. doi: 10.1371/journal.pntd.0005860. eCollection 2017 Aug. PLoS Negl Trop Dis. 2017. PMID: 28820881 Free PMC article.
-
Molecular Basis for Arbovirus Transmission by Aedes aegypti Mosquitoes.Intervirology. 2018;61(6):255-264. doi: 10.1159/000499128. Epub 2019 May 13. Intervirology. 2018. PMID: 31082816 Review.
Cited by
-
Three Immunocompetent Small Animal Models That Do Not Support Zika Virus Infection.Pathogens. 2021 Jul 30;10(8):971. doi: 10.3390/pathogens10080971. Pathogens. 2021. PMID: 34451435 Free PMC article.
-
A mosquito salivary protein-driven influx of myeloid cells facilitates flavivirus transmission.EMBO J. 2024 May;43(9):1690-1721. doi: 10.1038/s44318-024-00056-x. Epub 2024 Feb 20. EMBO J. 2024. PMID: 38378891 Free PMC article.
-
Saliva collection via capillary method may underestimate arboviral transmission by mosquitoes.Parasit Vectors. 2022 Mar 24;15(1):103. doi: 10.1186/s13071-022-05198-7. Parasit Vectors. 2022. PMID: 35331315 Free PMC article.
-
Trade-offs shaping transmission of sylvatic dengue and Zika viruses in monkey hosts.Nat Commun. 2024 Mar 27;15(1):2682. doi: 10.1038/s41467-024-46810-x. Nat Commun. 2024. PMID: 38538621 Free PMC article.
-
Characterization of bacteria expectorated during forced salivation of the Phlebotomus papatasi: A neglected component of sand fly infectious inoculums.PLoS Negl Trop Dis. 2024 May 21;18(5):e0012165. doi: 10.1371/journal.pntd.0012165. eCollection 2024 May. PLoS Negl Trop Dis. 2024. PMID: 38771858 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

