Multicentre Evaluation of Hepika Test Clinical Accuracy in Diagnosing HPV-Induced Cancer and Precancerous Lesions of the Uterine Cervix
- PMID: 33808260
- PMCID: PMC8066214
- DOI: 10.3390/diagnostics11040619
Multicentre Evaluation of Hepika Test Clinical Accuracy in Diagnosing HPV-Induced Cancer and Precancerous Lesions of the Uterine Cervix
Abstract
Objective: To evaluate the clinical accuracy of Hepika test to identify cancer/precancerous lesions of the uterine cervix.
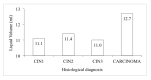
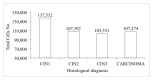
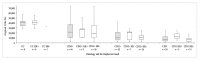
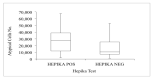
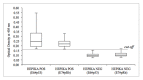
Materials and methods: A multicentre retrospective study was carried out in 2018 and included 330 liquid-based cytology samples from three Italian centres of women aged 25-64 who had been tested for the human papillomavirus (HPV) and whose histology or follow-up outcome was known. Hepika is an enzyme-linked immunosorbent assay (ELISA) targeting the protein complexes E6#p53 and E7#pRb. After excluding samples without sufficient residual material, the clinical accuracy of Hepika test was evaluated in 274 samples: adenocarcinoma (ADC) (4), squamous cell carcinoma (SCC) (7), adenocarcinoma in situ (AIS) (1), cervical intraepithelial neoplasia (CIN) grade 3 (60), CIN2 (51), CIN1 (34), and negative histology (117). Association, sensitivity, and specificity for carcinoma, CIN3+ and CIN2+ are reported.
Results: Positive Hepika test was associated with a high probability of carcinoma (odds ratio (DOR) = 33.68, 95% confidence interval (CI) 7.0-163.1); sensitivity was 81.8%, specificity, 88.2%. A positive Hepika test showed a weaker association with CIN3+ lesions (DOR = 3.5; 95% CI 1.75-6.99) and lower sensitivity (27.8%).
Conclusion: The Hepika test was found to be an accurate biomarker for HPV-induced cervical carcinoma. Population-based prospective studies are needed to confirm the clinical usefulness of the Hepika test in the differential diagnosis of HPV-induced invasive lesions.
Keywords: CIN; HPV; Hepika; cancer; carcinoma; precancerous lesion; tumor biomarker; uterine cervix.
Conflict of interest statement
The other authors declare no conflicts of interest.
Figures
Similar articles
-
p16 Immunohistochemistry is useful in confirming high-grade squamous intraepithelial lesions (HSIL) in women with negative HPV testing.Int J Gynecol Pathol. 2015 Mar;34(2):180-6. doi: 10.1097/PGP.0000000000000112. Int J Gynecol Pathol. 2015. PMID: 25675189
-
[The Clinical Significance of HPV L1/PD-L1 Tests Combined with Colposcopy for Cervical Precancerous Lesions and Cervical Cancer].Sichuan Da Xue Xue Bao Yi Xue Ban. 2021 May;52(3):516-522. doi: 10.12182/20210560105. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34018374 Free PMC article. Chinese.
-
Liquid-based cytology--new possibilities in the diagnosis of cervical lesions.Coll Antropol. 2010 Mar;34(1):19-24. Coll Antropol. 2010. PMID: 20432728
-
Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer.Vaccine. 2012 Nov 20;30 Suppl 5:F88-99. doi: 10.1016/j.vaccine.2012.06.095. Vaccine. 2012. PMID: 23199969 Review.
-
Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries.Vaccine. 2008 Aug 19;26 Suppl 10:K29-41. doi: 10.1016/j.vaccine.2008.06.019. Vaccine. 2008. PMID: 18847555 Review.
Cited by
-
Detection and Outcome of Endocervical Atypia in Cytology in Primary HPV Screening Programme.Diagnostics (Basel). 2021 Dec 20;11(12):2402. doi: 10.3390/diagnostics11122402. Diagnostics (Basel). 2021. PMID: 34943636 Free PMC article.
-
Diagnostic Value of Spiral CT and Magnetic Resonance Imaging Scanning in Gastric Cancer and Precancerous Lesions.Scanning. 2022 May 23;2022:3627385. doi: 10.1155/2022/3627385. eCollection 2022. Scanning. 2022. Retraction in: Scanning. 2023 Jun 21;2023:9896178. doi: 10.1155/2023/9896178. PMID: 35795615 Free PMC article. Retracted.
References
-
- WHO Human Papillomavirus (HPV) and Cervical Cancer. [(accessed on 30 January 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(h....
-
- Vizcaino A.P., Moreno V., Bosch F.X., Muñoz N., Barros-Dios X.M., Parkin D.M. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int. J. Cancer. 1998;75:536–545. doi: 10.1002/(SICI)1097-0215(19980209)75:4<536::AID-IJC8>3.0.CO;2-U. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

