The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice
- PMID: 33808318
- PMCID: PMC8036993
- DOI: 10.3390/molecules26071937
The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice
Abstract
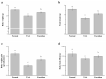
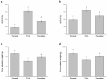
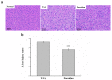
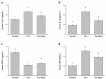
Liver disorders have been recognized as one major health concern. Fucoidan, a sulfated polysaccharide extracted from the brown seaweed Fucus serratus, has previously been reported as an anti-inflammatory and antioxidant. However, the discovery and validation of its hepatoprotective properties and elucidation of its mechanisms of action are still unknown. The objective of the current study was to investigate the effect and possible modes of action of a treatment of fucoidan against thioacetamide (TAA)-induced liver injury in male C57BL/6 mice by serum biochemical and histological analyses. The mouse model for liver damage was developed by the administration of TAA thrice a week for six weeks. The mice with TAA-induced liver injury were orally administered fucoidan once a day for 42 days. The treated mice showed significantly higher body weights; food intakes; hepatic antioxidative enzymes (catalase, glutathione peroxidase (GPx), and superoxide dismutase (SOD)); and a lower serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and C-reactive protein (CRP) levels. Additionally, a reduced hepatic IL-6 level and a decreased expression of inflammatory-related genes, such as cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) mRNA was observed. These results demonstrated that fucoidan had a hepatoprotective effect on liver injury through the suppression of the inflammatory responses and acting as an antioxidant. In addition, here, we validated the use of fucoidan against liver disorders with supporting molecular data.
Keywords: fucoidan; inflammation; liver; mice; thioacetamide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

