Probing the Interactions of Porphyrins with Macromolecules Using NMR Spectroscopy Techniques
- PMID: 33808335
- PMCID: PMC8037866
- DOI: 10.3390/molecules26071942
Probing the Interactions of Porphyrins with Macromolecules Using NMR Spectroscopy Techniques
Abstract
Porphyrinic compounds are widespread in nature and play key roles in biological processes such as oxygen transport in blood, enzymatic redox reactions or photosynthesis. In addition, both naturally derived as well as synthetic porphyrinic compounds are extensively explored for biomedical and technical applications such as photodynamic therapy (PDT) or photovoltaic systems, respectively. Their unique electronic structures and photophysical properties make this class of compounds so interesting for the multiple functions encountered. It is therefore not surprising that optical methods are typically the prevalent analytical tool applied in characterization and processes involving porphyrinic compounds. However, a wealth of complementary information can be obtained from NMR spectroscopic techniques. Based on the advantage of providing structural and dynamic information with atomic resolution simultaneously, NMR spectroscopy is a powerful method for studying molecular interactions between porphyrinic compounds and macromolecules. Such interactions are of special interest in medical applications of porphyrinic photosensitizers that are mostly combined with macromolecular carrier systems. The macromolecular surrounding typically stabilizes the encapsulated drug and may also modify its physical properties. Moreover, the interaction with macromolecular physiological components needs to be explored to understand and control mechanisms of action and therapeutic efficacy. This review focuses on such non-covalent interactions of porphyrinic drugs with synthetic polymers as well as with biomolecules such as phospholipids or proteins. A brief introduction into various NMR spectroscopic techniques is given including chemical shift perturbation methods, NOE enhancement spectroscopy, relaxation time measurements and diffusion-ordered spectroscopy. How these NMR tools are used to address porphyrin-macromolecule interactions with respect to their function in biomedical applications is the central point of the current review.
Keywords: NMR spectroscopy; cyclodextrin; drug delivery; interaction; micelles; nucleic acids; phospholipids; polymer; porphyrin; proteins; surfactant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



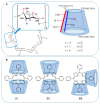


References
-
- Lemberg R. Porphyrins in Nature. In: Ƶechmeister L., editor. Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products/Progrés dans la Chimie des Substances Organiques Naturelles. Springer Vienna; Vienna, Austria: 1954. pp. 299–349.
-
- Williams R.J.P. The Properties Of Metalloporphyrins. Chem. Rev. 1956;56:299–328. doi: 10.1021/cr50008a004. - DOI
-
- Boucher L.J. Coordination Chemistry of Porphyrins. In: Melson G.A., editor. Coordination Chemistry of Macrocyclic Compounds. Springer US; Boston, MA, USA: 1979. pp. 517–536.
-
- Lesage S., Xu H.A.O., Durham L. The occurrence and roles of porphyrins in the environment: Possible implications for bioremediation. Hydrol. Sci. J. 1993;38:343–354. doi: 10.1080/02626669309492679. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

