ZnO Nanosheet-Coated TiZrPdSiNb Alloy as a Piezoelectric Hybrid Material for Self-Stimulating Orthopedic Implants
- PMID: 33808338
- PMCID: PMC8065972
- DOI: 10.3390/biomedicines9040352
ZnO Nanosheet-Coated TiZrPdSiNb Alloy as a Piezoelectric Hybrid Material for Self-Stimulating Orthopedic Implants
Abstract
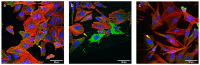
A Ti-based alloy (Ti45Zr15Pd30Si5Nb5) with already proven excellent mechanical and biocompatibility features has been coated with piezoelectric zinc oxide (ZnO) to induce the electrical self-stimulation of cells. ZnO was grown onto the pristine alloy in two different morphologies: a flat dense film and an array of nanosheets. The effect of the combined material on osteoblasts (electrically stimulable cells) was analyzed in terms of proliferation, cell adhesion, expression of differentiation markers and induction of calcium transients. Although both ZnO structures were biocompatible and did not induce inflammatory response, only the array of ZnO nanosheets was able to induce calcium transients, which improved the proliferation of Saos-2 cells and enhanced the expression of some early differentiation expression genes. The usual motion of the cells imposes strain to the ZnO nanosheets, which, in turn, create local electric fields owing to their piezoelectric character. These electric fields cause the opening of calcium voltage gates and boost cell proliferation and early differentiation. Thus, the modification of the Ti45Zr15Pd30Si5Nb5 surface with an array of ZnO nanosheets endows the alloy with smart characteristics, making it capable of electric self-stimulation.
Keywords: TiZrPdSiNb alloy; differentiation; nanogenerators; osteoblast; piezoelectric; proliferation; self-stimulating.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Alvarez K., Nakajima H. Metallic Scaffolds for Bone Regeneration. Materials. 2009;2:790–832. doi: 10.3390/ma2030790. - DOI
-
- Hynowska A., Blanquer A., Pellicer E., Fornell J., Suriñach S., Baro M.D., Gebert A., Calin M., Eckert J., Nogues C., et al. Nanostructured Ti-Zr-Pd-Si-(Nb) bulk metallic composites: Novel biocompatible materials with superior mechanical strength and elastic recovery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014;103:1569–1579. doi: 10.1002/jbm.b.33346. - DOI - PubMed
-
- Hynowska A., Pellicer E., Fornell J., González S., Van Steenberge N., Suriñach S., Gebert A., Calin M., Eckert J., Baró M.D., et al. Nanostructured β-phase Ti–31.0Fe–9.0Sn and sub-μm structured Ti–39.3Nb–13.3Zr–10.7Ta alloys for biomedical applications: Microstructure benefits on the mechanical and corrosion performances. Mater. Sci. Eng. C. 2012;32:2418–2425. doi: 10.1016/j.msec.2012.07.016. - DOI
Grants and funding
- 2017-SGR-292, 2017-SGR-503 and 2017-SGR-1420/Generalitat de Catalunya
- MAT2017-86357-C3-1-R (co-financed by the Fondo Europeo de Desarrollo Regional, FEDER), MAT2017-86357-C3-3-R and EUR2020-112082/Ministerio de Economía, Industria y Competitividad, Gobierno de España
- LCF/BQ/PR19/11700010/Fundación Bancaria Caixa d'Estalvis i Pensions de Barcelona
LinkOut - more resources
Full Text Sources
Other Literature Sources

