The Antiviral Activities of Poly-ADP-Ribose Polymerases
- PMID: 33808354
- PMCID: PMC8066025
- DOI: 10.3390/v13040582
The Antiviral Activities of Poly-ADP-Ribose Polymerases
Abstract
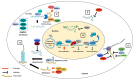
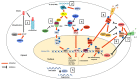
The poly-adenosine diphosphate (ADP)-ribose polymerases (PARPs) are responsible for ADP-ribosylation, a reversible post-translational modification involved in many cellular processes including DNA damage repair, chromatin remodeling, regulation of translation and cell death. In addition to these physiological functions, recent studies have highlighted the role of PARPs in host defenses against viruses, either by direct antiviral activity, targeting certain steps of virus replication cycle, or indirect antiviral activity, via modulation of the innate immune response. This review focuses on the antiviral activity of PARPs, as well as strategies developed by viruses to escape their action.
Keywords: PARP; antiviral; immunomodulation; viral escape mechanisms; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

