The Effect of Liquid Rubber Addition on the Physicochemical Properties, Cytotoxicity, and Ability to Inhibit Biofilm Formation of Dental Composites
- PMID: 33808411
- PMCID: PMC8038037
- DOI: 10.3390/ma14071704
The Effect of Liquid Rubber Addition on the Physicochemical Properties, Cytotoxicity, and Ability to Inhibit Biofilm Formation of Dental Composites
Abstract
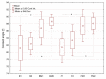
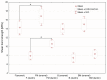
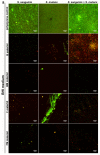
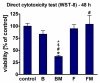
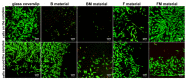
The aim of this study was to evaluate the effect of modification with liquid rubber on the adhesion to tooth tissues (enamel, dentin), wettability and ability to inhibit bacterial biofilm formation of resin-based dental composites. Two commercial composites (Flow-Art-flow type with 60% ceramic filler and Boston-packable type with 78% ceramic filler; both from Arkona Laboratorium Farmakologii Stomatologicznej, Nasutów, Poland) were modified by addition of 5% by weight (of resin) of a liquid methacrylate-terminated polybutadiene. Results showed that modification of the flow type composite significantly (p < 0.05) increased the shear bond strength values by 17% for enamel and by 33% for dentine. Addition of liquid rubber significantly (p < 0.05) reduced also hydrophilicity of the dental materials since the water contact angle was increased from 81-83° to 87-89°. Interestingly, modified packable type material showed improved antibiofilm activity against Steptococcus mutans and Streptococcus sanguinis (quantitative assay with crystal violet), but also cytotoxicity against eukaryotic cells since cell viability was reduced to 37% as proven in a direct-contact WST-8 test. Introduction of the same modification to the flow type material significantly improved its antibiofilm properties (biofilm reduction by approximately 6% compared to the unmodified material, p < 0.05) without cytotoxic effects against human fibroblasts (cell viability near 100%). Thus, modified flow type composite may be considered as a candidate to be used as restorative material since it exhibits both nontoxicity and antibiofilm properties.
Keywords: biofilm formation; cytotoxicity; resin composite; wettability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Improved Fracture Toughness and Conversion Degree of Resin-Based Dental Composites after Modification with Liquid Rubber.Materials (Basel). 2020 Jun 14;13(12):2704. doi: 10.3390/ma13122704. Materials (Basel). 2020. PMID: 32545845 Free PMC article.
-
Evaluation of dental adhesive systems incorporating an antibacterial monomer eugenyl methacrylate (EgMA) for endodontic restorations.Dent Mater. 2017 May;33(5):e239-e254. doi: 10.1016/j.dental.2017.01.016. Epub 2017 Feb 27. Dent Mater. 2017. PMID: 28245928
-
Biofilm formation on restorative materials and resin composite cements.Dent Mater. 2018 Nov;34(11):1702-1709. doi: 10.1016/j.dental.2018.08.300. Epub 2018 Sep 13. Dent Mater. 2018. PMID: 30220506
-
Adhesive luting of indirect restorations.Am J Dent. 2000 Nov;13(Spec No):60D-76D. Am J Dent. 2000. PMID: 11763920 Review.
-
The role of adhesive materials and oral biofilm in the failure of adhesive resin restorations.Am J Dent. 2017 Oct;30(5):285-292. Am J Dent. 2017. PMID: 29178733 Review.
Cited by
-
Study on Nitrile Oxide for Low-Temperature Curing of Liquid Polybutadiene.Materials (Basel). 2022 May 9;15(9):3396. doi: 10.3390/ma15093396. Materials (Basel). 2022. PMID: 35591729 Free PMC article.
-
Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review.Int J Mol Sci. 2023 Dec 21;25(1):152. doi: 10.3390/ijms25010152. Int J Mol Sci. 2023. PMID: 38203323 Free PMC article.
-
Miscibility and Optimization of the Liquid Rubber Content in the Resins of Light-Cured Dental Composites.Materials (Basel). 2022 Dec 22;16(1):87. doi: 10.3390/ma16010087. Materials (Basel). 2022. PMID: 36614425 Free PMC article.
-
Composite and Polymeric Materials for Dentistry: Enhancing Antimicrobial and Mechanical Properties.Materials (Basel). 2023 Feb 8;16(4):1432. doi: 10.3390/ma16041432. Materials (Basel). 2023. PMID: 36837061 Free PMC article.
References
-
- Moszner N., Salz U. Composites for dental restoratives. In: Shalaby W.S., Salz U., editors. Polymers for Dental and Orthopedic Applications. CRC Press; Boca Raton, FL, USA: 2006. pp. 13–68.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

