LDH and PDH Activities in the Ischemic Brain and the Effect of Reperfusion-An Ex Vivo MR Study in Rat Brain Slices Using Hyperpolarized [1-13C]Pyruvate
- PMID: 33808434
- PMCID: PMC8066106
- DOI: 10.3390/metabo11040210
LDH and PDH Activities in the Ischemic Brain and the Effect of Reperfusion-An Ex Vivo MR Study in Rat Brain Slices Using Hyperpolarized [1-13C]Pyruvate
Abstract
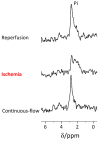
Ischemic stroke is a leading cause for neurologic disability worldwide, for which reperfusion is the only available treatment. Neuroimaging in stroke guides treatment, and therefore determines the clinical outcome. However, there are currently no imaging biomarkers for the status of the ischemic brain tissue. Such biomarkers could potentially be useful for guiding treatment in patients presenting with ischemic stroke. Hyperpolarized 13C MR of [1-13C]pyruvate is a clinically translatable method used to characterize tissue metabolism non-invasively in a relevant timescale. The aim of this study was to utilize hyperpolarized [1-13C]pyruvate to investigate the metabolic consequences of an ischemic insult immediately during reperfusion and upon recovery of the brain tissue. The rates of lactate dehydrogenase (LDH) and pyruvate dehydrogenase (PDH) were quantified by monitoring the rates of [1-13C]lactate and [13C]bicarbonate production from hyperpolarized [1-13C]pyruvate. 31P NMR of the perfused brain slices showed that this system is suitable for studying ischemia and recovery following reperfusion. This was indicated by the levels of the high-energy phosphates (tissue viability) and the chemical shift of the inorganic phosphate signal (tissue pH). Acidification, which was observed during the ischemic insult, has returned to baseline level following reperfusion. The LDH/PDH activity ratio increased following ischemia, from 47.0 ± 12.7 in the control group (n = 6) to 217.4 ± 121.3 in the ischemia-reperfusion group (n = 6). Following the recovery period (ca. 1.5 h), this value had returned to its pre-ischemia (baseline) level, suggesting the LDH/PDH enzyme activity ratio may be used as a potential indicator for the status of the ischemic and recovering brain.
Keywords: brain slices; dissolution dynamic nuclear polarization; ischemic stroke; lactate dehydrogenase; pyruvate dehydrogenase; reperfusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Feigin V.L., Nichols E., Alam T., Bannick M.S., Beghi E., Blake N., Culpepper W.J., Dorsey E.R., Elbaz A., Ellenbogen R.G., et al. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the global burden of disease study. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. - DOI - PMC - PubMed
-
- Feigin V.L., Forouzanfar M.H., Krishnamurthi R., Mensah G.A., Connor M., Bennett D.A., Moran A.E., Sacco R.L., Anderson L., Truelsen T., et al. Global and regional burden of stroke during 1990-2010: Findings from the global burden of disease study. Lancet. 2014;383:245–254. doi: 10.1016/S0140-6736(13)61953-4. - DOI - PMC - PubMed
-
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart disease and stroke statistics-2020 update: A report from the american heart association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. - DOI - PubMed
-
- Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

