Citrus Cell Suspension Culture Establishment, Maintenance, Efficient Transformation and Regeneration to Complete Transgenic Plant
- PMID: 33808465
- PMCID: PMC8066040
- DOI: 10.3390/plants10040664
Citrus Cell Suspension Culture Establishment, Maintenance, Efficient Transformation and Regeneration to Complete Transgenic Plant
Abstract
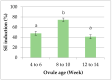
Agrobacterium-mediated transformation of epicotyl segment has been used in Citrus transgenic studies. The approach suffers, however, from limitations such as occasionally seed unavailability, the low transformation efficiency of juvenile tissues and the high frequency of chimeric plants. Therefore, a suspension cell culture system was established and used to generate transgenic plants in this study to overcome the shortcomings. The embryonic calli were successfully developed from undeveloped ovules of the three cultivars used in this study, "Sweet orange"-Egyptian cultivar (Citrus sinensis), "Shatangju" (Citrus reticulata) and "W. Murcott" (Citrus reticulata), on three different solid media. Effects of media, genotypes and ages of ovules on the induction of embryonic calli were also investigated. The result showed that the ovules' age interferes with the callus production more significantly than media and genotypes. The 8 to 10 week-old ovules were found to be the best materials. A cell suspension culture system was established in an H+H liquid medium. Transgenic plants were obtained from Agrobacterium-mediated transformation of cell suspension as long as eight weeks subculture intervals. A high transformation rate (~35%) was achieved by using our systems, confirming BASTA selection and later on by PCR confirmation. The results demonstrated that transformation of cell suspension should be more useful for the generation of non-chimeric transgenic Citrus plants. It was also shown that our cell suspension culture procedure was efficient in maintaining the vigor and regeneration potential of the cells.
Keywords: Citrus; cell suspension; de novo organogenesis; genetic engineering; tissue culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Zhong G., Nicolosi E. Citrus origin, diffusion, and economic importance. In: Gentile A., La Malfa S., Deng Z., editors. The Citrus Genome. Compendium of Plant Genomes. Springer; Cham, Switzerland: 2020. pp. 5–21.
-
- Moniruzzaman M., Zhong Y., Yan H., Yuanda L., Jiang B., Zhong G. Exploration of Susceptible Genes with Clustered Regularly Interspaced Short Palindromic Repeats–Tissue-Specific Knockout (CRISPR-TSKO) to Enhance Host Resistance. Crit. Rev. Plant Sci. 2020;39:387–417. doi: 10.1080/07352689.2020.1810970. - DOI
Grants and funding
- 2018B020202009/Guangdong Provincial Science and Technology Program
- 2019B030316005/Guangdong Provincial Science and Technology Program
- BZ201902/Dean's Foundation of Guangdong Academy of Agricultural Sciences
- 31601722/National Natural Sciences Foundation of China
- 32002016/National Natural Sciences Foundation of China
LinkOut - more resources
Full Text Sources
Other Literature Sources

