Stem Cells-Derived Extracellular Vesicles: Potential Therapeutics for Wound Healing in Chronic Inflammatory Skin Diseases
- PMID: 33808520
- PMCID: PMC8003197
- DOI: 10.3390/ijms22063130
Stem Cells-Derived Extracellular Vesicles: Potential Therapeutics for Wound Healing in Chronic Inflammatory Skin Diseases
Abstract
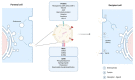
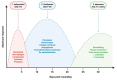
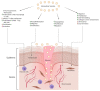
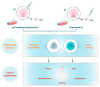
Endosome-derived small extracellular vesicles (EVs), often referred to as exosomes, are produced by almost all, if not all, cell types, and are critical for intercellular communication. They are composed of a lipid bilayer associated with membrane proteins and contain a payload of lipids, proteins and regulatory RNAs that depends on the parental cell physiological condition. By transferring their "cargo", exosomes can modulate the phenotype of neighboring and distant cells. Stem cells (SC) were widely studied for therapeutic applications regarding their regenerative/reparative potential as well as their immunomodulatory properties. Whether from autologous or allogeneic source, SC beneficial effects in terms of repair and regeneration are largely attributed to their paracrine signaling notably through secreted EVs. Subsequently, SC-derived EVs have been investigated for the treatment of various diseases, including inflammatory skin disorders, and are today fast-track cell-free tools for regenerative/reparative strategies. Yet, their clinical application is still facing considerable challenges, including production and isolation procedures, and optimal cell source. Within the emerging concept of "allogeneic-driven benefit" for SC-based therapies, the use of EVs from allogeneic sources becomes the pragmatic choice although a universal allogeneic cell source is still needed. As a unique temporary organ that ensures the mutual coexistence of two allogeneic organisms, mother and fetus, the human placenta offers a persuasive allogeneic stem cell source for development of therapeutic EVs. Advancing cell-free therapeutics nurtures great hope and provides new perspectives for the development of safe and effective treatment in regenerative/reparative medicine and beyond. We will outline the current state of the art in regard of EVs, summarize their therapeutic potential in the context of skin inflammatory disorders, and discuss their translational advantages and hurdles.
Keywords: chronic inflammation; exosomes; extracellular vesicles; regenerative medicine; skin; stem cells; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Extracellular Vesicles in Cardiovascular Theranostics.Theranostics. 2017 Sep 26;7(17):4168-4182. doi: 10.7150/thno.21274. eCollection 2017. Theranostics. 2017. PMID: 29158817 Free PMC article. Review.
-
Stem cell- derived extracellular vesicles as new tools in regenerative medicine - Immunomodulatory role and future perspectives.Front Immunol. 2023 Jan 24;14:1120175. doi: 10.3389/fimmu.2023.1120175. eCollection 2023. Front Immunol. 2023. PMID: 36761725 Free PMC article. Review.
-
On the Choice of the Extracellular Vesicles for Therapeutic Purposes.Int J Mol Sci. 2019 Jan 9;20(2):236. doi: 10.3390/ijms20020236. Int J Mol Sci. 2019. PMID: 30634425 Free PMC article. Review.
-
Focus on Extracellular Vesicles: Therapeutic Potential of Stem Cell-Derived Extracellular Vesicles.Int J Mol Sci. 2016 Feb 6;17(2):174. doi: 10.3390/ijms17020174. Int J Mol Sci. 2016. PMID: 26861305 Free PMC article. Review.
-
Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine.Cells. 2020 Mar 13;9(3):707. doi: 10.3390/cells9030707. Cells. 2020. PMID: 32183102 Free PMC article. Review.
Cited by
-
CD80 Antibody and MTX Co-Engineered Extracellular Vesicles Targets CD80+ Macrophages to Suppress Inflammation and Alleviate Chronic Inflammatory Diseases.Int J Nanomedicine. 2025 May 21;20:6379-6398. doi: 10.2147/IJN.S517357. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40416732 Free PMC article.
-
Bone marrow-derived mesenchymal stromal cells in necrotizing enterocolitis treatment: a narrative review.Front Pediatr. 2025 Jul 31;13:1624236. doi: 10.3389/fped.2025.1624236. eCollection 2025. Front Pediatr. 2025. PMID: 40822681 Free PMC article. Review.
-
Frontier Review of the Molecular Mechanisms and Current Approaches of Stem Cell-Derived Exosomes.Cells. 2023 Mar 26;12(7):1018. doi: 10.3390/cells12071018. Cells. 2023. PMID: 37048091 Free PMC article. Review.
-
Mussel-inspired sulfated hyaluronan cryogel patch with antioxidant, anti-inflammatory, and drug-loading properties for multifunctional wound adhesives.Bioact Mater. 2024 Aug 14;40:582-596. doi: 10.1016/j.bioactmat.2024.08.001. eCollection 2024 Oct. Bioact Mater. 2024. PMID: 39239260 Free PMC article.
-
Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Diabetic Wound Healing via Promoting M2 Macrophage Polarization, Angiogenesis, and Collagen Deposition.Int J Mol Sci. 2022 Sep 9;23(18):10421. doi: 10.3390/ijms231810421. Int J Mol Sci. 2022. PMID: 36142334 Free PMC article.
References
-
- Boukouaci W., Lauden L., Siewiera J., Dam N., Hocine H.R., Khaznadar Z., Tamouza R., Borlado L.R., Charron D., Jabrane-Ferrat N., et al. Natural killer cell crosstalk with allogeneic human cardiac-derived stem/progenitor cells controls persistence. Cardiovasc Res. 2014;104:290–302. doi: 10.1093/cvr/cvu208. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

