Dopamine D3 Receptor Plasticity in Parkinson's Disease and L-DOPA-Induced Dyskinesia
- PMID: 33808538
- PMCID: PMC8003204
- DOI: 10.3390/biomedicines9030314
Dopamine D3 Receptor Plasticity in Parkinson's Disease and L-DOPA-Induced Dyskinesia
Abstract
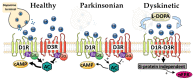
Parkinson's Disease (PD) is characterized by primary and secondary plasticity that occurs in response to progressive degeneration and long-term L-DOPA treatment. Some of this plasticity contributes to the detrimental side effects associated with chronic L-DOPA treatment, namely L-DOPA-induced dyskinesia (LID). The dopamine D3 receptor (D3R) has emerged as a promising target in LID management as it is upregulated in LID. This upregulation occurs primarily in the D1-receptor-bearing (D1R) cells of the striatum, which have been repeatedly implicated in LID manifestation. D3R undergoes dynamic changes both in PD and in LID, making it difficult to delineate D3R's specific contributions, but recent genetic and pharmacologic tools have helped to clarify its role in LID. The following review will discuss these changes, recent advances to better clarify D3R in both PD and LID and potential steps for translating these findings.
Keywords: D1R–D3R; L-DOPA-induced dyskinesia; Parkinson’s Disease; dopamine D1 receptor; dopamine D3 receptor; striatum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Genetic suppression of the dopamine D3 receptor in striatal D1 cells reduces the development of L-DOPA-induced dyskinesia.Exp Neurol. 2021 Feb;336:113534. doi: 10.1016/j.expneurol.2020.113534. Epub 2020 Nov 27. Exp Neurol. 2021. PMID: 33249031
-
5alpha-reductase inhibitors dampen L-DOPA-induced dyskinesia via normalization of dopamine D1-receptor signaling pathway and D1-D3 receptor interaction.Neurobiol Dis. 2019 Jan;121:120-130. doi: 10.1016/j.nbd.2018.09.018. Epub 2018 Sep 24. Neurobiol Dis. 2019. PMID: 30261284
-
Dopamine D3 Receptor Modulates l-DOPA-Induced Dyskinesia by Targeting D1 Receptor-Mediated Striatal Signaling.Cereb Cortex. 2017 Jan 1;27(1):435-446. doi: 10.1093/cercor/bhv231. Cereb Cortex. 2017. PMID: 26483399 Free PMC article.
-
Dopamine receptors: homomeric and heteromeric complexes in L-DOPA-induced dyskinesia.J Neural Transm (Vienna). 2018 Aug;125(8):1187-1194. doi: 10.1007/s00702-018-1852-x. Epub 2018 Feb 7. J Neural Transm (Vienna). 2018. PMID: 29417335 Review.
-
Neurobiological and Pharmacological Perspectives of D3 Receptors in Parkinson's Disease.Biomolecules. 2022 Feb 1;12(2):243. doi: 10.3390/biom12020243. Biomolecules. 2022. PMID: 35204744 Free PMC article. Review.
Cited by
-
Neurophysiological treatment effects of mesdopetam, pimavanserin and clozapine in a rodent model of Parkinson's disease psychosis.Neurotherapeutics. 2024 Mar;21(2):e00334. doi: 10.1016/j.neurot.2024.e00334. Epub 2024 Feb 16. Neurotherapeutics. 2024. PMID: 38368170 Free PMC article.
-
Adopting the Rumsfeld approach to understanding the action of levodopa and apomorphine in Parkinson's disease.J Neural Transm (Vienna). 2023 Nov;130(11):1337-1347. doi: 10.1007/s00702-023-02655-0. Epub 2023 May 20. J Neural Transm (Vienna). 2023. PMID: 37210460 Free PMC article. Review.
-
Neurophysiological Treatment Effects of Mesdopetam, Pimavanserin and Amantadine in a Rodent Model of Levodopa-Induced Dyskinesia.Eur J Neurosci. 2025 Mar;61(5):e70032. doi: 10.1111/ejn.70032. Eur J Neurosci. 2025. PMID: 40042199 Free PMC article.
-
Dopamine Signaling in Substantia Nigra and Its Impact on Locomotor Function-Not a New Concept, but Neglected Reality.Int J Mol Sci. 2024 Jan 17;25(2):1131. doi: 10.3390/ijms25021131. Int J Mol Sci. 2024. PMID: 38256204 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

