Compendium of Methods to Uncover RNA-Protein Interactions In Vivo
- PMID: 33808611
- PMCID: PMC8006020
- DOI: 10.3390/mps4010022
Compendium of Methods to Uncover RNA-Protein Interactions In Vivo
Abstract
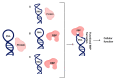
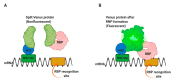
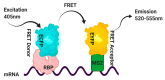
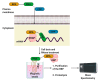
Control of gene expression is critical in shaping the pro-and eukaryotic organisms' genotype and phenotype. The gene expression regulatory pathways solely rely on protein-protein and protein-nucleic acid interactions, which determine the fate of the nucleic acids. RNA-protein interactions play a significant role in co- and post-transcriptional regulation to control gene expression. RNA-binding proteins (RBPs) are a diverse group of macromolecules that bind to RNA and play an essential role in RNA biology by regulating pre-mRNA processing, maturation, nuclear transport, stability, and translation. Hence, the studies aimed at investigating RNA-protein interactions are essential to advance our knowledge in gene expression patterns associated with health and disease. Here we discuss the long-established and current technologies that are widely used to study RNA-protein interactions in vivo. We also present the advantages and disadvantages of each method discussed in the review.
Keywords: RNA; RNA–protein interactions; gene expression and post-transcriptional gene regulation; ribonucleoproteins.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

