Role of HO-1 against Saturated Fatty Acid-Induced Oxidative Stress in Hepatocytes
- PMID: 33808635
- PMCID: PMC8003531
- DOI: 10.3390/nu13030993
Role of HO-1 against Saturated Fatty Acid-Induced Oxidative Stress in Hepatocytes
Abstract
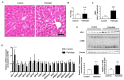
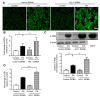
Increased circulating levels of free fatty acids, especially saturated ones, are involved in disease progression in the non-alcoholic fatty liver. Although the mechanism of saturated fatty acid-induced toxicity in the liver is not fully understood, oxidative stress may be deeply involved. We examined the effect of increased palmitic acid, the most common saturated fatty acid in the blood, on the liver of BALB/c mice via tail vein injection with palmitate. After 24 h, among several anti-oxidative stress response genes, only heme oxygenase-1 (HO-1) was significantly upregulated in palmitate-injected mice compared with that in vehicle-injected mice. Elevation of HO-1 mRNA was also observed in the fatty liver of high-fat-diet-fed mice. To further investigate the role of HO-1 on palmitic acid-induced oxidative stress, in vitro experiments were performed to expose palmitate to HepG2 cells. SiRNA-mediated knockdown of HO-1 significantly increased the oxidative stress induced by palmitate, whereas pre-treatment with SnCl2, a well-known HO-1 inducer, significantly decreased it. Moreover, SB203580, a selective p38 inhibitor, reduced HO-1 mRNA expression and increased palmitate-induced oxidative stress in HepG2 cells. These results suggest that the HO-1-mediated anti-oxidative stress compensatory reaction plays an essential role against saturated fatty acid-induced lipotoxicity in the liver.
Keywords: fatty liver; hem oxygenase-1; lipotoxicity; oxidative stress; palmitic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

