Melanin Distribution in Human Skin: Influence of Cytoskeletal, Polarity, and Centrosome-Related Machinery of Stratum basale Keratinocytes
- PMID: 33808676
- PMCID: PMC8003549
- DOI: 10.3390/ijms22063143
Melanin Distribution in Human Skin: Influence of Cytoskeletal, Polarity, and Centrosome-Related Machinery of Stratum basale Keratinocytes
Abstract
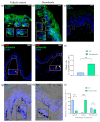
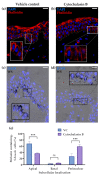
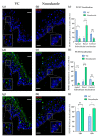
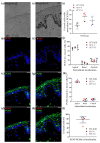
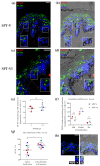
Melanin granules cluster within supra-nuclear caps in basal keratinocytes (KCs) of the human epidermis, where they protect KC genomic DNA against ultraviolet radiation (UVR) damage. While much is known about melanogenesis in melanocytes (MCs) and a moderate amount about melanin transfer from MC to KC, we know little about the fate of melanin once inside KCs. We recently reported that melanin fate in progenitor KCs is regulated by rare asymmetric organelle movement during mitosis. Here, we explore the role of actin, microtubules, and centrosome-associated machinery in distributing melanin within KCs. Short-term cultures of human skin explants were treated with cytochalasin-B and nocodazole to target actin filaments and microtubules, respectively. Treatment effects on melanin distribution were assessed by the Warthin-Starry stain, on centrosome-associated proteins by immunofluorescence microscopy, and on co-localisation with melanin granules by brightfield microscopy. Cytochalasin-B treatment disassembled supra-nuclear melanin caps, while nocodazole treatment moved melanin from the apical to basal KC domain. Centrosome and centriolar satellite-associated proteins showed a high degree of co-localisation with melanin. Thus, once melanin granules are transferred to KCs, their preferred apical distribution appears to be facilitated by coordinated movement of centrosomes and centriolar satellites. This mechanism may control melanin's strategic position within UVR-exposed KCs.
Keywords: Stratum basale keratinocytes; actin; centriolar satellites; centrosome; epidermis; ex vivo human skin; melanin distribution; microtubules; skin phototype.
Conflict of interest statement
M.B. and C.O.’C. are employees of Walgreen-Boots Alliance (UK).
Figures

References
-
- Kobayashi N., Nakagawa A., Muramatsu T., Yamashina Y., Shirai T., Hashimoto M.W., Ishigaki Y., Ohnishi T., Mori T. Supranuclear Melanin Caps Reduce Ultraviolet Induced DNA Photoproducts in Human Epidermis. J. Investig. Dermatol. 1998;110:806–810. doi: 10.1046/j.1523-1747.1998.00178.x. - DOI - PubMed
-
- Fajuyigbe D., Lwin S.M., Diffey B.L., Baker R., Tobin D.J., Sarkany R.P.E., Young A.R. Melanin distribution in human epidermis affords localized protection against DNA photodamage and concurs with skin cancer incidence difference in extreme phototypes. FASEB J. 2018;32:3700–3706. doi: 10.1096/fj.201701472R. - DOI - PubMed
-
- Joly-Tonetti N., Wibawa J.I.D., Bell M., Tobin D.J. An explanation for the mysterious distribution of melanin in human skin—A rare example of asymmetric (melanin) organelle distribution during mitosis of basal layer progenitor keratinocytes. Br. J. Dermatol. 2018;179 doi: 10.1111/bjd.16926. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

