Aromatic Amino Acid Decarboxylase Deficiency: The Added Value of Biochemistry
- PMID: 33808712
- PMCID: PMC8003434
- DOI: 10.3390/ijms22063146
Aromatic Amino Acid Decarboxylase Deficiency: The Added Value of Biochemistry
Abstract
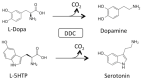
Aromatic amino acid decarboxylase (AADC) deficiency is a rare, autosomal recessive neurometabolic disorder caused by mutations in the DDC gene, leading to a deficit of AADC, a pyridoxal 5'-phosphate requiring enzyme that catalyzes the decarboxylation of L-Dopa and L-5-hydroxytryptophan in dopamine and serotonin, respectively. Although clinical and genetic studies have given the major contribution to the diagnosis and therapy of AADC deficiency, biochemical investigations have also helped the comprehension of this disorder at a molecular level. Here, we reported the steps leading to the elucidation of the functional and structural features of the enzyme that were useful to identify the different molecular defects caused by the mutations, either in homozygosis or in heterozygosis, associated with AADC deficiency. By revisiting the biochemical data available on the characterization of the pathogenic variants in the purified recombinant form, and interpreting them on the basis of the structure-function relationship of AADC, it was possible: (i) to define the enzymatic phenotype of patients harboring pathogenic mutations and at the same time to propose specific therapeutic managements, and (ii) to identify residues and/or regions of the enzyme relevant for catalysis and/or folding of AADC.
Keywords: AADC deficiency; aromatic amino acid decarboxylase; dopa decarboxylase; pathogenic variants; pyridoxal 5′-phophate; rare disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
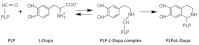

Similar articles
-
Heterozygosis in aromatic amino acid decarboxylase deficiency: Evidence for a positive interallelic complementation between R347Q and R358H mutations.IUBMB Life. 2018 Mar;70(3):215-223. doi: 10.1002/iub.1718. Epub 2018 Jan 22. IUBMB Life. 2018. PMID: 29356298
-
A novel compound heterozygous genotype associated with aromatic amino acid decarboxylase deficiency: Clinical aspects and biochemical studies.Mol Genet Metab. 2019 Jun;127(2):132-137. doi: 10.1016/j.ymgme.2019.05.004. Epub 2019 May 10. Mol Genet Metab. 2019. PMID: 31104889
-
Prevalence of Aromatic l-Amino Acid Decarboxylase Deficiency in At-Risk Populations.Pediatr Neurol. 2020 May;106:38-42. doi: 10.1016/j.pediatrneurol.2019.11.022. Epub 2019 Dec 26. Pediatr Neurol. 2020. PMID: 32111562
-
Aromatic amino acid decarboxylase deficiency: Molecular and metabolic basis and therapeutic outlook.Mol Genet Metab. 2019 May;127(1):12-22. doi: 10.1016/j.ymgme.2019.03.009. Epub 2019 Mar 27. Mol Genet Metab. 2019. PMID: 30952622 Review.
-
Compound Heterozygosis in AADC Deficiency and Its Complex Phenotype in Terms of AADC Protein Population.Int J Mol Sci. 2022 Sep 23;23(19):11238. doi: 10.3390/ijms231911238. Int J Mol Sci. 2022. PMID: 36232540 Free PMC article. Review.
Cited by
-
Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases.Int J Mol Sci. 2025 Mar 23;26(7):2916. doi: 10.3390/ijms26072916. Int J Mol Sci. 2025. PMID: 40243512 Free PMC article. Review.
-
Elucidating the Interaction between Pyridoxine 5'-Phosphate Oxidase and Dopa Decarboxylase: Activation of B6-Dependent Enzyme.Int J Mol Sci. 2022 Dec 30;24(1):642. doi: 10.3390/ijms24010642. Int J Mol Sci. 2022. PMID: 36614085 Free PMC article.
-
Aromatic L-Amino Acid Decarboxylase Deficiency: A Genetic Screening in Sicilian Patients with Neurological Disorders.Genes (Basel). 2024 Jan 21;15(1):134. doi: 10.3390/genes15010134. Genes (Basel). 2024. PMID: 38275615 Free PMC article.
-
Molecular signature of stem-like glioma cells (SLGCs) from human glioblastoma and gliosarcoma.PLoS One. 2024 Feb 2;19(2):e0291368. doi: 10.1371/journal.pone.0291368. eCollection 2024. PLoS One. 2024. PMID: 38306361 Free PMC article.
-
The Integrated Approach to Inherited Disorders in Neurotransmitters from Molecules to Systems.Int J Mol Sci. 2022 Dec 15;23(24):15974. doi: 10.3390/ijms232415974. Int J Mol Sci. 2022. PMID: 36555620 Free PMC article.
References
-
- Pearson T.S., Gilbert L., Opladen T., Garcia-Cazorla A., Mastrangelo M., Leuzzi V., Tay S.K.H., Sykut-Cegielska J., Pons R., Mercimek-Andrews S., et al. AADC deficiency from infancy to adulthood: Symptoms and developmental outcome in an international cohort of 63 pa-tients. J. Inherit. Metab. Dis. 2020;43:1121–1130. doi: 10.1002/jimd.12247. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

