Graphene Oxide-Silver Nanoparticle Nanocomposites Induce Oxidative Stress and Aberrant Methylation in Caprine Fetal Fibroblast Cells
- PMID: 33808775
- PMCID: PMC8003532
- DOI: 10.3390/cells10030682
Graphene Oxide-Silver Nanoparticle Nanocomposites Induce Oxidative Stress and Aberrant Methylation in Caprine Fetal Fibroblast Cells
Abstract
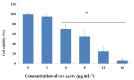
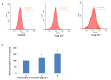
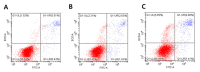
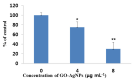
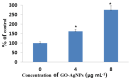
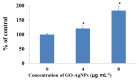
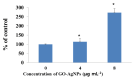
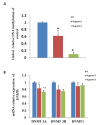
Graphene oxide-silver nanoparticle (GO-AgNPs) nanocomposites have drawn much attention for their potential in biomedical uses. However, the potential toxicity of GO-AgNPs in animals and humans remains unknown, particularly in the developing fetus. Here, we reported the GO-AgNP-mediated cytotoxicity and epigenetic alteration status in caprine fetal fibroblast cells (CFFCs). In brief, the proliferation and apoptosis rate of GO-AgNP-treated CFFCs (4 and 8 µg/mL of GO-AgNPs) were measured using the cell-counting kit (CCK-8) assay and the annexin V/propidium iodide (PI) assay, respectively. In addition, the oxidative stress induced by GO-AgNPs and detailed mechanisms were studied by evaluating the generation of reactive oxygen species (ROS), superoxide dismutase (SOD), lactate dehydrogenase (LDH), malondialdehyde (MDA), and caspase-3 and abnormal methylation. The expression of pro- and anti-apoptotic genes and DNA methyltransferases was measured using reverse transcription followed by RT-qPCR. Our data indicated that GO-AgNPs cause cytotoxicity in a dose-dependent manner. GO-AgNPs induced significant cytotoxicity by the loss of cell viability, production of ROS, increasing leakage of LDH and level of MDA, increasing expression of pro-apoptotic genes, and decreasing expression of anti-apoptotic genes. GO-AgNPs incited DNA hypomethylation and the decreased expression of DNMT3A. Taken together, this study showed that GO-AgNPs increase the generation of ROS and cause apoptosis and DNA hypomethylation in CFFCs. Therefore, the potential applications of GO-AgNPs in biomedicine should be re-evaluated.
Keywords: caprine fetal fibroblast cells (CFFCs); epigenetic; graphene oxide; reactive oxygen species (ROS); silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

