Oral Manifestations in Melanoma Patients Treated with Target or Immunomodulatory Therapies
- PMID: 33808846
- PMCID: PMC8003791
- DOI: 10.3390/jcm10061283
Oral Manifestations in Melanoma Patients Treated with Target or Immunomodulatory Therapies
Abstract
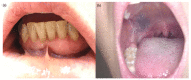
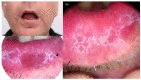
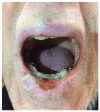
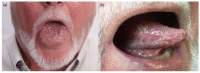
BRAF (v-raf murine sarcoma viral oncogene homolog B1) and MEK (mitogen activated protein kinase) inhibitors, as well as immunotherapy against cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and the programmed cell death 1 (PD-1) receptor and its ligand (PD-L1), have shown good results in improving the disease-free survival of patients with metastatic melanoma (MM). The aim of this review is to summarize the main oral adverse events (oAEs) occurring in patients undergoing target or immunotherapy. We proposed two separate sections: oAEs during the treatment with (1) target therapies with BRAF and MEK inhibitors and tyrosine kinase inhibitors (gingival hyperplasia, pigmentation disorders, squamo-proliferative lesions) and (2) immunotherapies with CTLA-4 or PD1 inhibitors (lichenoid reactions, immuno-bullous reactions, xerostomia and other reactions). Adverse events frequently include oAEs, although these are often misdiagnosed and under-reported. Indeed, the oral cavity is not routinely evaluated during clinical practice. The symptomatology related to oAEs is significant since it may represent the first manifestation of a severe systemic reaction, possibly leading to difficulties in nutrition with a consequent impact on patients' quality of life. A careful examination of the oral cavity is recommended during the evaluation of oncologic patients in order to promptly detect the onset of new manifestations.
Keywords: adverse event; immunotherapy; melanoma; oral; target.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nava C., Hanna N., Michot C., Pereira S., Pouvreau N., Niihori T., Aoki Y., Matsubara Y., Arveiler B., Lacombe D., et al. Cardio-facio-cutaneous and Noonan syndromes due to mutations in the RAS/MAPK signalling pathway: Genotype phenotype relationships and overlap with Costello syndrome. J. Med Genet. 2007;44:763–771. doi: 10.1136/jmg.2007.050450. - DOI - PMC - PubMed
-
- Gripp K.W., Lin A.E., Nicholson L., Allen W., Cramer A., Jones K.L., Kutz W., Peck D., Rebolledo M.A., Wheeler P.G., et al. Further delineation of the phenotype resulting from BRAF or MEK1 germline mutations helps differentiate cardio-facio-cutaneous syndrome from Costello syndrome. Am. J. Med Genet. Part A. 2007;143:1472–1480. doi: 10.1002/ajmg.a.31815. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

