Evaluation of Typhoid Conjugate Vaccine Effectiveness in Ghana (TyVEGHA) Using a Cluster-Randomized Controlled Phase IV Trial: Trial Design and Population Baseline Characteristics
- PMID: 33808924
- PMCID: PMC8003794
- DOI: 10.3390/vaccines9030281
Evaluation of Typhoid Conjugate Vaccine Effectiveness in Ghana (TyVEGHA) Using a Cluster-Randomized Controlled Phase IV Trial: Trial Design and Population Baseline Characteristics
Abstract
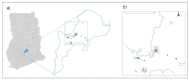
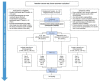
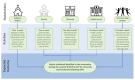
Typhoid fever remains a significant health problem in sub-Saharan Africa, with incidence rates of >100 cases per 100,000 person-years of observation. Despite the prequalification of safe and effective typhoid conjugate vaccines (TCV), some uncertainties remain around future demand. Real-life effectiveness data, which inform public health programs on the impact of TCVs in reducing typhoid-related mortality and morbidity, from an African setting may help encourage the introduction of TCVs in high-burden settings. Here, we describe a cluster-randomized trial to investigate population-level protection of TYPBAR-TCV®, a Vi-polysaccharide conjugated to a tetanus-toxoid protein carrier (Vi-TT) against blood-culture-confirmed typhoid fever, and the synthesis of health economic evidence to inform policy decisions. A total of 80 geographically distinct clusters are delineated within the Agogo district of the Asante Akim region in Ghana. Clusters are randomized to the intervention arm receiving Vi-TT or a control arm receiving the meningococcal A conjugate vaccine. The primary study endpoint is the total protection of Vi-TT against blood-culture-confirmed typhoid fever. Total, direct, and indirect protection are measured as secondary outcomes. Blood-culture-based enhanced surveillance enables the estimation of incidence rates in the intervention and control clusters. Evaluation of the real-world impact of TCVs and evidence synthesis improve the uptake of prequalified/licensed safe and effective typhoid vaccines in public health programs of high burden settings. This trial is registered at the Pan African Clinical Trial Registry, accessible at Pan African Clinical Trials Registry (ID: PACTR202011804563392).
Keywords: Ghana; cluster randomized trial; typhoid conjugate vaccine; typhoid fever.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Mogasale V., Maskery B., Ochiai R.L., Lee J.S., Mogasale V.V., Ramani E., Kim Y.K., Wierzba T.F. Burden of typhoid fever in low-income and middle-income countries: A systematic, literature-based update with risk-factor adjustment. Lancet Glob. Health. 2014;2:e570–e580. doi: 10.1016/S2214-109X(14)70301-8. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

