Progression of Metastasis through Lymphatic System
- PMID: 33808959
- PMCID: PMC7999434
- DOI: 10.3390/cells10030627
Progression of Metastasis through Lymphatic System
Abstract
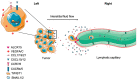
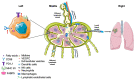
Lymph nodes are the most common sites of metastasis in cancer patients. Nodal disease status provides great prognostic power, but how lymph node metastases should be treated is under debate. Thus, it is important to understand the mechanisms by which lymph node metastases progress and how they can be targeted to provide therapeutic benefits. In this review, we focus on delineating the process of cancer cell migration to and through lymphatic vessels, survival in draining lymph nodes and further spread to other distant organs. In addition, emerging molecular targets and potential strategies to inhibit lymph node metastasis are discussed.
Keywords: anti-tumor immunity; lymph node; lymphatic vessel; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

