Holospiniferoside: A New Antitumor Cerebroside from The Red Sea Cucumber Holothuria spinifera: In Vitro and In Silico Studies
- PMID: 33809026
- PMCID: PMC8001240
- DOI: 10.3390/molecules26061555
Holospiniferoside: A New Antitumor Cerebroside from The Red Sea Cucumber Holothuria spinifera: In Vitro and In Silico Studies
Abstract
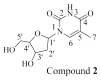
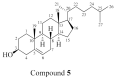
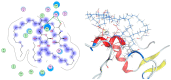
Chemical investigation of the methanolic extract of the Red Sea cucumber Holothuria spinifera led to the isolation of a new cerebroside, holospiniferoside (1), together with thymidine (2), methyl-α-d-glucopyranoside (3), a new triacylglycerol (4), and cholesterol (5). Their chemical structures were established by NMR and mass spectrometric analysis, including gas chromatography-mass spectrometry (GC-MS) and high-resolution mass spectrometry (HRMS). All the isolated compounds are reported in this species for the first time. Moreover, compound 1 exhibited promising in vitro antiproliferative effect on the human breast cancer cell line (MCF-7) with IC50 of 20.6 µM compared to the IC50 of 15.3 µM for the drug cisplatin. To predict the possible mechanism underlying the cytotoxicity of compound 1, a docking study was performed to elucidate its binding interactions with the active site of the protein Mdm2-p53. Compound 1 displayed an apoptotic activity via strong interaction with the active site of the target protein. This study highlights the importance of marine natural products in the design of new anticancer agents.
Keywords: HRMS; Holothuria spinifera; cerebrosides; cytotoxicity; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Elhady S.S., Eltamany E.E., Shaaban A.E., Bagalagel A.A., Muhammad Y.A., El-Sayed N.M., Ayyad S.-E.N., Ahmed A.A.M., Elgawish M.S., Ahmed S.A. Jaceidin flavonoid isolated from Chiliadenus montanus attenuates tumor progression in mice via VEGF inhibition: In Vivo and in silico studies. Plants. 2020;9:1031. doi: 10.3390/plants9081031. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

