Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis
- PMID: 33809062
- PMCID: PMC8001566
- DOI: 10.3390/md19030148
Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis
Abstract
Non-alcoholic fatty liver disease (NAFLD) is the emerging cause of chronic liver disease globally and lack of approved therapies. Here, we investigated the feasibility of combinatorial effects of low molecular weight fucoidan and high stability fucoxanthin (LMF-HSFx) as a therapeutic approach against NAFLD. We evaluated the inhibitory effects of LMF-HSFx or placebo in 42 NAFLD patients for 24 weeks and related mechanism in high fat diet (HFD) mice model and HepaRGTM cell line. We found that LMF-HSFx reduces the relative values of alanine aminotransferase, aspartate aminotransferase, total cholesterol, triglyceride, fasting blood glucose and hemoglobin A1c in NAFLD patients. For lipid metabolism, LMF-HSFx reduces the scores of controlled attenuation parameter (CAP) and increases adiponectin and leptin expression. Interestingly, it reduces liver fibrosis in NAFLD patients, either. The proinflammatory cytokines interleukin (IL)-6 and interferon-γ are reduced in LMF-HSFx group. In HFD mice, LMF-HSFx attenuates hepatic lipotoxicity and modulates adipogenesis. Additionally, LMF-HSFx modulates SIRI-PGC-1 pathway in HepaRG cells under palmitic acid-induced lipotoxicity environment. Here, we describe that LMF-HSFx ameliorated hepatic steatosis, inflammation, fibrosis and insulin resistance in NAFLD patients. LMF-HSFx may modulate leptin-adiponectin axis in adipocytes and hepatocytes, then regulate lipid and glycogen metabolism, decrease insulin resistance and is against NAFLD.
Keywords: NAFLD; adiponectin; lipid metabolism; lipotoxicity; liver fibrosis; randomized controlled trial.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
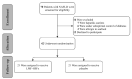
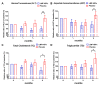

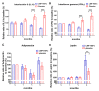
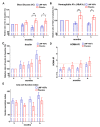
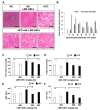
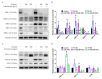
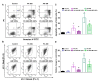
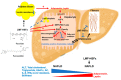
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

