Novel Multifunctional Ascorbic Triazole Derivatives for Amyloidogenic Pathway Inhibition, Anti-Inflammation, and Neuroprotection
- PMID: 33809092
- PMCID: PMC7999550
- DOI: 10.3390/molecules26061562
Novel Multifunctional Ascorbic Triazole Derivatives for Amyloidogenic Pathway Inhibition, Anti-Inflammation, and Neuroprotection
Abstract
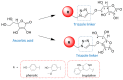
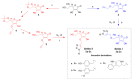
Alzheimer's disease (AD) is a common neurodegenerative disorder. The number of patients with AD is projected to reach 152 million by 2050. Donepezil, rivastigmine, galantamine, and memantine are the only four drugs currently approved by the United States Food and Drug Administration for AD treatment. However, these drugs can only alleviate AD symptoms. Thus, this research focuses on the discovery of novel lead compounds that possess multitarget regulation of AD etiopathology relating to amyloid cascade. The ascorbic acid structure has been designated as a core functional domain due to several characteristics, including antioxidant activities, amyloid aggregation inhibition, and the ability to be transported to the brain and neurons. Multifunctional ascorbic derivatives were synthesized by copper (I)-catalyzed azide-alkyne cycloaddition reaction (click chemistry). The in vitro and cell-based assays showed that compounds 2c and 5c exhibited prominent multifunctional activities as beta-secretase 1 inhibitors, amyloid aggregation inhibitors, and antioxidant, neuroprotectant, and anti-inflammatory agents. Significant changes in activities promoting neuroprotection and anti-inflammation were observed at a considerably low concentration at a nanomolar level. Moreover, an in silico study showed that compounds 2c and 5c were capable of being permeated across the blood-brain barrier by sodium-dependent vitamin C transporter-2.
Keywords: BACE1 inhibitor; amyloid aggregation inhibition; anti-inflammation; antioxidant; ascorbic derivatives; neuroprotective.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Dementia Statistics. Alzheimer’s Disease International. [(accessed on 4 February 2021)]; Available online: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics.
-
- Yatin S.M., Yatin M., Aulick T., Ain K.B., Butterfield D.A. Alzheimer’s amyloid beta-peptide associated free radicals increase rat embryonic neuronal polyamine uptake and ornithine decarboxylase activity: Protective effect of vitamin E. Neurosci. Lett. 1999;263:17–20. doi: 10.1016/S0304-3940(99)00101-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

