KLF15 Loss-of-Function Mutation Underlying Atrial Fibrillation as well as Ventricular Arrhythmias and Cardiomyopathy
- PMID: 33809104
- PMCID: PMC8001991
- DOI: 10.3390/genes12030408
KLF15 Loss-of-Function Mutation Underlying Atrial Fibrillation as well as Ventricular Arrhythmias and Cardiomyopathy
Abstract
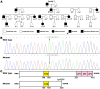
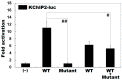
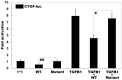
Atrial fibrillation (AF) represents the most common type of clinical cardiac arrhythmia and substantially increases the risks of cerebral stroke, heart failure and death. Accumulating evidence has convincingly demonstrated the strong genetic basis of AF, and an increasing number of pathogenic variations in over 50 genes have been causally linked to AF. Nevertheless, AF is of pronounced genetic heterogeneity, and the genetic determinants underpinning AF in most patients remain obscure. In the current investigation, a Chinese pedigree with AF as well as ventricular arrhythmias and hypertrophic cardiomyopathy was recruited. Whole exome sequencing and bioinformatic analysis of the available family members were conducted, and a novel heterozygous variation in the KLF15 gene (encoding Krüppel-like factor 15, a transcription factor critical for cardiac electrophysiology and structural remodeling), NM_014079.4: c.685A>T; p.(Lys229*), was identified. The variation was verified by Sanger sequencing and segregated with autosomal dominant AF in the family with complete penetrance. The variation was absent from 300 unrelated healthy subjects used as controls. In functional assays using a dual-luciferase assay system, mutant KLF15 showed neither transcriptional activation of the KChIP2 promoter nor transcriptional inhibition of the CTGF promoter, alone or in the presence of TGFB1, a key player in the pathogenesis of arrhythmias and cardiomyopathies. The findings indicate KLF15 as a new causative gene responsible for AF as well as ventricular arrhythmias and hypertrophic cardiomyopathy, and they provide novel insight into the molecular mechanisms underlying cardiac arrhythmias and hypertrophic cardiomyopathy.
Keywords: KLF15; arrhythmia; atrial fibrillation; cardiomyopathy; genetics; transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Miyazaki S., Kajiyama T., Yamao K., Hada M., Yamaguchi M., Nakamura H., Hachiya H., Tada H., Hirao K., Iesaka Y. Silent cerebral events/lesions after second-generation cryoballoon ablation: How can we reduce the risk of silent strokes? Heart Rhythm. 2019;16:41–48. doi: 10.1016/j.hrthm.2018.07.011. - DOI - PubMed
-
- Graves K.G., May H.T., Jacobs V., Knowlton K.U., Muhlestein J.B., Lappe D.L., Anderson J.L., Horne B.D., Bunch T.J. CHA(2)DS(2)-VASc scores and Intermountain Mortality Risk Scores for the joint risk stratification of dementia among patients with atrial fibrillation. Heart Rhythm. 2019;16:3–9. doi: 10.1016/j.hrthm.2018.10.018. - DOI - PubMed
-
- Bunch T.J., Bair T.L., Crandall B.G., Cutler M.J., Day J.D., Graves K.G., Jacobs V., Mallender C., Osborn J.S., Weiss J.P., et al. Stroke and dementia risk in patients with and without atrial fibrillation and carotid arterial disease. Heart Rhythm. 2020;17:20–26. doi: 10.1016/j.hrthm.2019.07.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

