A Descriptive-Multivariate Analysis of Community Knowledge, Confidence, and Trust in COVID-19 Clinical Trials among Healthcare Workers in Uganda
- PMID: 33809269
- PMCID: PMC8000597
- DOI: 10.3390/vaccines9030253
A Descriptive-Multivariate Analysis of Community Knowledge, Confidence, and Trust in COVID-19 Clinical Trials among Healthcare Workers in Uganda
Abstract
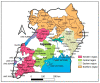
Background-misinformation and mistrust often undermines community vaccine uptake, yet information in rural communities, especially of developing countries, is scarce. This study aimed to identify major challenges associated with coronavirus disease 2019 (COVID-19) vaccine clinical trials among healthcare workers and staff in Uganda. Methods-a rapid exploratory survey was conducted over 5 weeks among 260 respondents (66% male) from healthcare centers across the country using an online questionnaire. Twenty-seven questions assessed knowledge, confidence, and trust scores on COVID-19 vaccine clinical trials from participants in 46 districts in Uganda. Results-we found low levels of knowledge (i.e., confusing COVID-19 with Ebola) with males being more informed than females (OR = 1.5, 95% CI: 0.7-3.0), and mistrust associated with policy decisions to promote herbal treatments in Uganda and the rushed international clinical trials, highlighting challenges for the upcoming Oxford-AstraZeneca vaccinations. Knowledge, confidence and trust scores were higher among the least educated (certificate vs. bachelor degree holders). We also found a high level of skepticism and possible community resistance to DNA recombinant vaccines, such as the Oxford-AstraZeneca vaccine. Preference for herbal treatments (38/260; 14.6%, 95% CI: 10.7-19.3) currently being promoted by the Ugandan government raises major policy concerns. High fear and mistrust for COVID-19 vaccine clinical trials was more common among wealthier participants and more affluent regions of the country. Conclusion-our study found that knowledge, confidence, and trust in COVID-19 vaccines was low among healthcare workers in Uganda, especially those with higher wealth and educational status. There is a need to increase transparency and inclusive participation to address these issues before new trials of COVID-19 vaccines are initiated.
Keywords: COVAX; COVID-19; COVID-19 and medical workers; COVID-19 clinical trials in resource poor countries; clinical trials in Africa; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kennedy S.B., Neaton J.D., Lane H.C., Kieh M.W.S., Massaquoi M.B.F., Touchette N.A., Nason M.C., Follmann D.A., Boley F.K., Johnson M.P., et al. Implementation of an Ebola virus disease vaccine clinical trial during the Ebola epidemic in Liberia: Design, procedures, and challenges. Clin. Trials. 2016;13:49–56. doi: 10.1177/1740774515621037. - DOI - PubMed
-
- Enria L., Lees S., Smout E., Mooney T., Tengbeh A.F., Leigh B., Greenwood B., Watson-Jones D., Larson H. Power, fairness and trust: Understanding and engaging with vaccine trial participants and communities in the setting up the EBOVAC-Salone vaccine trial in Sierra Leone. BMC Public Health. 2016;16:1–10. doi: 10.1186/s12889-016-3799-x. - DOI - PMC - PubMed
-
- Erku D.A., Belachew S.A., Abrha S., Sinnollareddy M., Thomas J., Steadman K.J., Tesfaye W.H. When fear and misinformation go viral: Pharmacists’ role in deterring medication misinformation during the ’infodemic’ surrounding COVID-19. Res. Soc. Adm. Pharm. 2021;17:1954–1963. doi: 10.1016/j.sapharm.2020.04.032. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

