Issues and Controversies in the Evolution of Oral Rehydration Therapy (ORT)
- PMID: 33809275
- PMCID: PMC8005945
- DOI: 10.3390/tropicalmed6010034
Issues and Controversies in the Evolution of Oral Rehydration Therapy (ORT)
Erratum in
-
Correction: Nalin, D. Issues and Controversies in the Evolution of Oral Rehydration Therapy (ORT). Trop. Med. Infect. Dis. 2021, 6, 34.Trop Med Infect Dis. 2022 Jun 14;7(6):103. doi: 10.3390/tropicalmed7060103. Trop Med Infect Dis. 2022. PMID: 35736991 Free PMC article.
Abstract
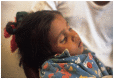
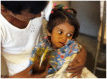
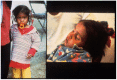
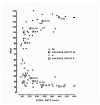
The original studies demonstrating the efficacy of oral glucose-electrolytes solutions in reducing or eliminating the need for intravenous therapy to correct dehydration caused by acute watery diarrheas (AWD) were focused chiefly on cholera patients. Later research adapted the oral therapy (ORT) methodology for treatment of non-cholera AWDs including for pediatric patients. These adaptations included the 2:1 regimen using 2 parts of the original WHO oral rehydration solution (ORS) formulation followed by 1 part additional plain water, and a "low sodium" packet formulation with similar average electrolyte and glucose concentrations when dissolved in the recommended volume of water. The programmatic desire for a single ORS packet formulation has led to controversy over use of the "low sodium" formulations to treat cholera patients. This is the subject of the current review, with the conclusion that use of the low-sodium ORS to treat cholera patients leads to negative sodium balance, leading to hyponatremia and, in severe cases, particularly in pediatric cholera, to seizures and other complications of sodium depletion. Therefore it is recommended that two separate ORS packet formulations be used, one for cholera therapy and the other for non-cholera pediatric AWD.
Keywords: ORS formulations; cholera; hyponatremia; hyponatremic seizures; hyponatremic sequelae; non-cholera acute watery diarrheas (AWDs); oral rehydration solutions (ORS); sodium balance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Current status of oral rehydration as a strategy for the control of diarrhoeal diseases.Indian J Med Res. 1996 Jul;104:115-24. Indian J Med Res. 1996. PMID: 8783513 Review.
-
History of development of oral rehydration therapy.Indian J Public Health. 1994 Apr-Jun;38(2):39-43. Indian J Public Health. 1994. PMID: 7530695
-
Role of oral rehydration therapy in controlling epidemic of cholera and watery diarrhoea.J Indian Med Assoc. 2003 Jun;101(6):379-80, 386. J Indian Med Assoc. 2003. PMID: 14579986
-
Comparison of low and high sodium and potassium content in oral rehydration solutions.J Pediatr. 1980 Nov;97(5):848-53. doi: 10.1016/s0022-3476(80)80287-3. J Pediatr. 1980. PMID: 7431183 Clinical Trial.
-
Clinical trials of improved oral rehydration salt formulations: a review.Bull World Health Organ. 1994;72(6):945-55. Bull World Health Organ. 1994. PMID: 7867142 Free PMC article. Review.
Cited by
-
The History of Intravenous and Oral Rehydration and Maintenance Therapy of Cholera and Non-Cholera Dehydrating Diarrheas: A Deconstruction of Translational Medicine: From Bench to Bedside?Trop Med Infect Dis. 2022 Mar 12;7(3):50. doi: 10.3390/tropicalmed7030050. Trop Med Infect Dis. 2022. PMID: 35324597 Free PMC article. Review.
-
Using Oral Rehydration Therapy (ORT) in the Community.Trop Med Infect Dis. 2021 May 29;6(2):92. doi: 10.3390/tropicalmed6020092. Trop Med Infect Dis. 2021. PMID: 34072599 Free PMC article.
-
Correction: Nalin, D. Issues and Controversies in the Evolution of Oral Rehydration Therapy (ORT). Trop. Med. Infect. Dis. 2021, 6, 34.Trop Med Infect Dis. 2022 Jun 14;7(6):103. doi: 10.3390/tropicalmed7060103. Trop Med Infect Dis. 2022. PMID: 35736991 Free PMC article.
-
Water pollution, cholera, and the role of probiotics: a comprehensive review in relation to public health in Bangladesh.Front Microbiol. 2025 Jan 14;15:1523397. doi: 10.3389/fmicb.2024.1523397. eCollection 2024. Front Microbiol. 2025. PMID: 39877756 Free PMC article. Review.
-
Recent surge in cholera outbreaks globally during the COVID-19 pandemic era: a potential threat to the African continent and salient counteracting strategies.Int J Surg. 2023 Mar 1;109(3):631-633. doi: 10.1097/JS9.0000000000000222. Int J Surg. 2023. PMID: 36912513 Free PMC article. No abstract available.
References
-
- WHO Reduced Osmolarity Oral Rehydration Salts (ORS) Formulation; Department of Child and Adolescent Health and Development. [(accessed on 20 October 2020)]. Available online: https://apps.who.int/iris/handle/10665/67322.
-
- Alam S., Afzal K., Maheshwari M., Shukla I. Controlled trial of hypo-osmolar versus world Health Organization Oral rehydration solution. Indian Pediatrics. 2000;37:952–960. - PubMed
-
- Pulungsih S.P., Punjabi N.H., Rafli K., Rifajati A., Kumala S., Simanjuntak C.H., Yuwono Lesmana M., Subekti D., Sutoto, Fontaine O. Standard WHO_ORS versus reduced-osmolarity ORS in the management of cholera patients. J. Health Popul. Nutr. 2006;24:107–112. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

