Vitamin D and Cardiovascular Disease: An Updated Narrative Review
- PMID: 33809311
- PMCID: PMC7998446
- DOI: 10.3390/ijms22062896
Vitamin D and Cardiovascular Disease: An Updated Narrative Review
Abstract
During the last two decades, the potential impact of vitamin D on the risk of cardiovascular disease (CVD) has been rigorously studied. Data regarding the effect of vitamin D on CVD risk are puzzling: observational data indicate an inverse nonlinear association between vitamin D status and CVD events, with the highest CVD risk at severe vitamin D deficiency; however, preclinical data and randomized controlled trials (RCTs) show several beneficial effects of vitamin D on the surrogate parameters of vascular and cardiac function. By contrast, Mendelian randomization studies and large RCTs in the general population and in patients with chronic kidney disease, a high-risk group for CVD events, largely report no significant beneficial effect of vitamin D treatment on CVD events. In patients with rickets and osteomalacia, cardiovascular complications are infrequently reported, except for an increased risk of heart failure. In conclusion, there is no strong evidence for beneficial vitamin D effects on CVD risk, either in the general population or in high-risk groups. Whether some subgroups such as individuals with severe vitamin D deficiency or a combination of low vitamin D status with specific gene variants and/or certain nutrition/lifestyle factors would benefit from vitamin D (metabolite) administration, remains to be studied.
Keywords: atherosclerosis; calcium; cardiovascular; chronic kidney disease; epidemiology; heart; mortality; vitamin D; vitamin D receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
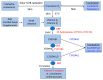
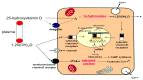
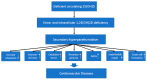
References
-
- Jahreis G., Hesse V. Vitamin D-induced tissue calcinosis and arteriosclerosis changes. I: A contribution to the 60-year history of vitamin D research with special reference to childhood. Padiatr. Grenzgeb. 1990;29:203–211. (In German) - PubMed
-
- Holick M.F. Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight Do We Need? Adv. Exp. Med. Biol. 2020;1268:19–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

