Interleukins as Mediators of the Tumor Cell-Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis
- PMID: 33809315
- PMCID: PMC7999500
- DOI: 10.3390/ijms22062898
Interleukins as Mediators of the Tumor Cell-Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis
Abstract
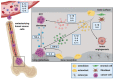
Patients with advanced breast cancer are at high risk of developing bone metastasis. Despite treatment advances for primary breast cancer, metastatic bone disease remains incurable with a low relative survival. Hence, new therapeutic approaches are required to improve survival and treatment outcome for these patients. Bone is among the most frequent sites of metastasis in breast cancer. Once in the bone, disseminated tumor cells can acquire a dormant state and remain quiescent until they resume growth, resulting in overt metastasis. At this stage the disease is characterized by excessive, osteoclast-mediated osteolysis. Cells of the bone microenvironment including osteoclasts, osteoblasts and endothelial cells contribute to the initiation and progression of breast cancer bone metastasis. Direct cell-to-cell contact as well as soluble factors regulate the crosstalk between disseminated breast cancer cells and bone cells. In this complex signaling network interleukins (ILs) have been identified as key regulators since both, cancer cells and bone cells secrete ILs and express corresponding receptors. ILs regulate differentiation and function of bone cells, with several ILs being reported to act pro-osteoclastogenic. Consistently, the expression level of ILs (e.g., in serum) has been associated with poor prognosis in breast cancer. In this review we discuss the role of the most extensively investigated ILs during the establishment of breast cancer bone metastasis and highlight their potential as therapeutic targets in preventing metastatic outgrowth in bone.
Keywords: bone metastasis; bone microenvironment; breast cancer; disseminated tumor cell; dormancy; interleukin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pathological Crosstalk between Metastatic Breast Cancer Cells and the Bone Microenvironment.Biomolecules. 2020 Feb 19;10(2):337. doi: 10.3390/biom10020337. Biomolecules. 2020. PMID: 32092997 Free PMC article. Review.
-
What Is Breast in the Bone?Int J Mol Sci. 2016 Oct 22;17(10):1764. doi: 10.3390/ijms17101764. Int J Mol Sci. 2016. PMID: 27782069 Free PMC article. Review.
-
Endogenous Production of IL1B by Breast Cancer Cells Drives Metastasis and Colonization of the Bone Microenvironment.Clin Cancer Res. 2019 May 1;25(9):2769-2782. doi: 10.1158/1078-0432.CCR-18-2202. Epub 2019 Jan 22. Clin Cancer Res. 2019. PMID: 30670488
-
Location matters: osteoblast and osteoclast distribution is modified by the presence and proximity to breast cancer cells in vivo.Clin Exp Metastasis. 2012 Dec;29(8):927-38. doi: 10.1007/s10585-012-9481-5. Epub 2012 May 6. Clin Exp Metastasis. 2012. PMID: 22562502
-
Direct crosstalk between cancer and osteoblast lineage cells fuels metastatic growth in bone via auto-amplification of IL-6 and RANKL signaling pathways.J Bone Miner Res. 2014 Sep;29(9):1938-49. doi: 10.1002/jbmr.2231. J Bone Miner Res. 2014. PMID: 24676805
Cited by
-
Feed-forward loops between metastatic cancer cells and their microenvironment-the stage of escalation.EMBO Mol Med. 2022 Jun 8;14(6):e14283. doi: 10.15252/emmm.202114283. Epub 2022 May 4. EMBO Mol Med. 2022. PMID: 35506376 Free PMC article. Review.
-
Subcutaneous fat predicts bone metastasis in breast cancer: A novel multimodality-based deep learning model.Cancer Biomark. 2024;39(3):171-185. doi: 10.3233/CBM-230219. Cancer Biomark. 2024. PMID: 38043007 Free PMC article.
-
Anterior Gradient 2 is a Significant Prognostic Biomarker in Bone Metastasis of Breast Cancer.Pathol Oncol Res. 2022 Nov 3;28:1610538. doi: 10.3389/pore.2022.1610538. eCollection 2022. Pathol Oncol Res. 2022. PMID: 36405393 Free PMC article.
-
Radiomics analysis of thoracic vertebral bone marrow microenvironment changes before bone metastasis of breast cancer based on chest CT.J Bone Oncol. 2024 Nov 26;50:100653. doi: 10.1016/j.jbo.2024.100653. eCollection 2025 Feb. J Bone Oncol. 2024. PMID: 39712652 Free PMC article.
-
Diagnosis of SPECT/CT bone imaging combined with two serum examinations in patients with bone metastases from pulmonary cancer.Clin Transl Oncol. 2024 Jan;26(1):147-154. doi: 10.1007/s12094-023-03231-4. Epub 2023 Jun 3. Clin Transl Oncol. 2024. PMID: 37269491
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

