Modulation of the Biocatalytic Properties of a Novel Lipase from Psychrophilic Serratia sp. (USBA-GBX-513) by Different Immobilization Strategies
- PMID: 33809323
- PMCID: PMC8001504
- DOI: 10.3390/molecules26061574
Modulation of the Biocatalytic Properties of a Novel Lipase from Psychrophilic Serratia sp. (USBA-GBX-513) by Different Immobilization Strategies
Abstract
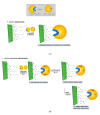
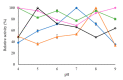
In this work, the effect of different immobilization procedures on the properties of a lipase obtained from the extremophilic microorganism Serratia sp. USBA-GBX-513, which was isolated from Paramo soils of Los Nevados National Natural Park (Colombia), is reported. Different Shepharose beads were used: octyl-(OC), octyl-glyoxyl-(OC-GLX), cyanogen bromide (BrCN)-, and Q-Sepharose. The performance of the different immobilized extremophile lipase from Serratia (ESL) was compared with that of the lipase B from Candida antarctica (CALB). In all immobilization tests, hyperactivation of ESL was observed. The highest hyperactivation (10.3) was obtained by immobilization on the OC support. Subsequently, the thermal stability at pH 5, 7, and 9 and the stability in the presence of 50% (v/v) acetonitrile, 50% dioxane, and 50% tetrahydrofuran solvents at pH 7 and 40 °C were evaluated. ESL immobilized on octyl-Sepharose was the most stable biocatalyst at 90 °C and pH 9, while the most stable preparation at pH 5 was ESL immobilized on OC-GLX-Sepharose supports. Finally, in the presence of 50% (v/v) tetrahydrofuran (THF) or dioxane at 40 °C, ESL immobilized on OC-Sepharose was the most stable biocatalyst, while the immobilized preparation of ESL on Q-Sepharose was the most stable one in 40% (v/v) acetonitrile.
Keywords: USBA-GBX-513; hyperactivation; immobilization; interfacial activation; lipase from psychrophilic microorganism.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Oliart-Ros R., Manresa-Presas A., Sánchez-Otero M. Utilization of microorganisms from extreme environments and their products in biotechnological development. CienciaUAT. 2016;11:79–90. doi: 10.29059/cienciauat.v11i1.556. - DOI
-
- Bharathi D., Rajalakshmi G. Microbial lipases: An overview of screening, production and purification. Biocatal. Agric. Biotechnol. 2019;22:101368. doi: 10.1016/j.bcab.2019.101368. - DOI
-
- Mateo C., Palomo J.M., Fernandez-Lorente G., Guisan J.M., Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007;40:1451–1463. doi: 10.1016/j.enzmictec.2007.01.018. - DOI
-
- Iyer P.V., Ananthanarayan L. Enzyme stability and stabilization-Aqueous and non-aqueous environment. Process Biochem. 2008;43:1019–1032. doi: 10.1016/j.procbio.2008.06.004. - DOI
-
- García-Galán C., Berenguer-Murcia Á., Fernández-Lafuente R., Rodrígues R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011;353:2885–2904. doi: 10.1002/adsc.201100534. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

