Effect of Acetylation on Physicochemical and Functional Properties of Commercial Pumpkin Protein Concentrate
- PMID: 33809328
- PMCID: PMC8002035
- DOI: 10.3390/molecules26061575
Effect of Acetylation on Physicochemical and Functional Properties of Commercial Pumpkin Protein Concentrate
Abstract
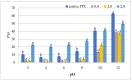
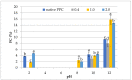
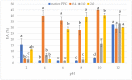
The purpose of the present study was to determine the effects of acetylation with different doses of acetic anhydride on the chemical composition and chosen functional properties of commercial pumpkin protein concentrate (PPC). The total protein content decreased as compared to unmodified samples. Electrophoretic analysis revealed that in the acetylated pumpkin protein, the content of the heaviest protein (35 kDa) decreased in line with increasing concentrations of modifying reagent. Acetylation of PPC caused a significant increase in water-binding and oil-absorption capacity and for emulsifying properties even at the dose of 0.4 mL/g. Additionally, an increase in foaming capacity was demonstrated for preparations obtained with 2.0 mL/g of acetic anhydride, whereas acetylation with 0.4 and 1.0 mL/g caused a decrease in protein solubility as compared to native PPC.
Keywords: SDS-PAGE; acetylation; amino acid profile; chemical composition; functional properties; in vitro digestibility; pumpkin protein concentrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Antoniewska A., Adamska A., Rutkowska J., Zielińska M. Olej z nasion dyni jako źródło cennych składników w diecie człowieka. Probl. Hig. Epidemiol. 2017;98:17–22. (In Polish)
-
- Murkovic M. Pumpkin seed oil. In: Moreau R.A., Kamal-Eldin A., editors. Gourmet and Health—Promoting Specialty Oils. 1st ed. Academic Press; Cambridge, MA, USA: AOCS Press; Urbana, IL, USA: 2009. pp. 345–358.
-
- Peričin D., Radulović-Popović L., Vaštag Ž., Mađarev-Popović S., Trivić S. Enymatic hydrolysis of protein isolate from hull-less pumpkin oil cake: Application of response surface methodology. Food Chem. 2009;115:753–757. doi: 10.1016/j.foodchem.2008.12.040. - DOI
-
- Vaštag Ž., Popović L., Popović S., Krimer V., Peričin D. Production of enzymatic hydrolysates with antioxidant and angiotensin-I converting enzyme inhibitory activity from pumpkin oil cake protein isolate. Food Chem. 2011;124:1316–1321. doi: 10.1016/j.foodchem.2010.07.062. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

