The Vps13 Family of Lipid Transporters and Its Role at Membrane Contact Sites
- PMID: 33809364
- PMCID: PMC7999203
- DOI: 10.3390/ijms22062905
The Vps13 Family of Lipid Transporters and Its Role at Membrane Contact Sites
Abstract
The conserved VPS13 proteins constitute a new family of lipid transporters at membrane contact sites. These large proteins are suspected to bridge membranes and form a direct channel for lipid transport between organelles. Mutations in the 4 human homologs (VPS13A-D) are associated with a number of neurological disorders, but little is known about their precise functions or the relevant contact sites affected in disease. In contrast, yeast has a single Vps13 protein which is recruited to multiple organelles and contact sites. The yeast model system has proved useful for studying the function of Vps13 at different organelles and identifying the localization determinants responsible for its membrane targeting. In this review we describe recent advances in our understanding of VPS13 proteins with a focus on yeast research.
Keywords: Atg2; Cohen syndrome; Parkinson’s disease; Vps13; ataxia; chorea acanthocytosis; lipid transport; membrane contact sites; yeast model.
Conflict of interest statement
The authors declare no conflict of interest.
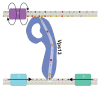
Figures


References
-
- Nishimura A.L., Mitne-Neto M., Silva H.C.A., Richieri-Costa A., Middleton S., Cascio D., Kok F., Oliveira J.R.M., Gillingwater T., Webb J., et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 2004;75:822–831. doi: 10.1086/425287. - DOI - PMC - PubMed
-
- Bryant D., Liu Y., Datta S., Hariri H., Seda M., Anderson G., Peskett E., Demetriou C., Sousa S., Jenkins D., et al. SNX14 mutations affect endoplasmic reticulum-associated neutral lipid metabolism in autosomal recessive spinocerebellar ataxia 20. Hum. Mol. Genet. 2018;27:1927–1940. doi: 10.1093/hmg/ddy101. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

