Rolipram Prevents the Formation of Abdominal Aortic Aneurysm (AAA) in Mice: PDE4B as a Target in AAA
- PMID: 33809405
- PMCID: PMC8000788
- DOI: 10.3390/antiox10030460
Rolipram Prevents the Formation of Abdominal Aortic Aneurysm (AAA) in Mice: PDE4B as a Target in AAA
Abstract
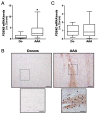
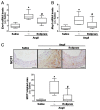
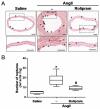
Abdominal aortic aneurysm (AAA) is a common life-threatening condition characterized by exacerbated inflammation and the generation of reactive oxygen species. Pharmacological treatments to slow AAA progression or to prevent its rupture remain a challenge. Targeting phosphodiesterase 4 (PDE4) has been verified as an effective therapeutic strategy for an array of inflammatory conditions; however, no studies have assessed yet PDE4 in AAA. Here, we used angiotensin II (AngII)-infused apolipoprotein E deficient mice to study the involvement of the PDE4 subfamily in aneurysmal disease. PDE4B but not PDE4D was upregulated in inflammatory cells from both experimental and human AAA. The administration of the PDE4 selective inhibitor rolipram (3 mg/kg/day) to AngII-challenged mice (1000 ng/kg bodyweight/min) protected against AAA formation, limiting the progressive increase in the aortic diameter without affecting the blood pressure. The drug strongly attenuated the rise in vascular oxidative stress (superoxide anion) induced by AngII, and decreased the expression of inflammatory markers, as well as the recruitment of macrophages (MAC3+), lymphocytes (CD3+), and neutrophils (ELANE+) into the vessel wall. Rolipram also normalized the vascular MMP2 expression and MMP activity, preserving the elastin integrity and improving the vascular remodelling. These results point to PDE4B as a new therapeutic target for AAA.
Keywords: PDE4B; abdominal aortic aneurysm; reactive oxygen species; rolipram.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
-
- Chaikof E.L., Dalman R.L., Eskandari M.K., Jackson B.M., Lee W.A., Mansour M.A., Mastracci T.M., Mell M., Murad M.H., Nguyen L.L., et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018;67:2–77.e2. doi: 10.1016/j.jvs.2017.10.044. - DOI - PubMed
Grants and funding
- PI18/0919/Instituto de Salud Carlos III
- RTI2018-094727-B-100/Ministerio de Ciencia e Innovación
- PID2019-108489RB-100/Ministerio de Ciencia e Innovación
- 2017-SGR-00333/Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR)
- Beca FEA 2020 Investigación Básica/Sociedad Española de Arteriosclerosis
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

