Possible Relevance of Soluble Luteinizing Hormone Receptor during Development and Adulthood in Boys and Men
- PMID: 33809538
- PMCID: PMC7999540
- DOI: 10.3390/cancers13061329
Possible Relevance of Soluble Luteinizing Hormone Receptor during Development and Adulthood in Boys and Men
Abstract
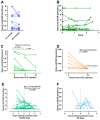
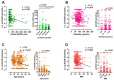
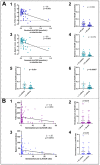
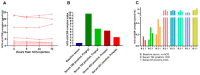
Luteinizing hormone (LH) and human chorionic gonadotropin (hCG) are agonists for the luteinizing hormone receptor (LHCGR) which regulates male reproductive function. LHCGR may be released into body fluids. We wish to determine whether soluble LHCGR is a marker for gonadal function. Cross-sectional, longitudinal, and intervention studies on 195 healthy boys and men and 396 men with infertility, anorchia, or Klinefelter Syndrome (KS) were used to correlate LHCGR measured in serum, seminal fluid, urine, and hepatic/renal artery and vein with gonadal function. LHCGR was determined in fluids from in vitro and in vivo models of human testicular tissue and cell lines, xenograft mouse models, and human fetal kidney and adrenal glands. Western blot showed LHCGR fragments in serum and gonadal tissue of similar size using three different antibodies. The LHCGR-ELISA had no species cross-reactivity or unspecific reaction in mouse serum even after human xenografting. Instead, sLHCGR was released into the media after the culture of a human fetal kidney and adrenal glands. Serum sLHCGR decreased markedly during puberty in healthy boys (p = 0.0001). In healthy men, serum sLHCGR was inversely associated with the Inhibin B/FSH ratio (β -0.004, p = 0.027). In infertile men, seminal fluid sLHCGR was inversely associated with serum FSH (β 0.006, p = 0.009), sperm concentration (β -3.5, p = 0.003) and total sperm count (β -3.2, p = 0.007). The injection of hCG lowered sLHCGR in serum and urine of healthy men (p < 0.01). In conclusion, sLHCGR is released into body-fluids and linked with pubertal development and gonadal function. Circulating sLHCGR in anorchid men suggests that sLHCGR in serum may originate from and possibly exert actions in non-gonadal tissues. (ClinicalTrials: NTC01411527, NCT01304927, NCT03418896).
Keywords: LH receptor; NTera2; TCAM2; development; extra-gonadal effects; fetal adrenal gland; fetal kidney; gonadotropins; infertility; puberty.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

