Wheat Varietal Response to Tilletia controversa J. G. Kühn Using qRT-PCR and Laser Confocal Microscopy
- PMID: 33809560
- PMCID: PMC8000713
- DOI: 10.3390/genes12030425
Wheat Varietal Response to Tilletia controversa J. G. Kühn Using qRT-PCR and Laser Confocal Microscopy
Abstract
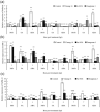
Tilletia controversa J. G. Kühn is a causal organism of dwarf bunt in wheat. Understanding the interaction of wheat and T. controversa is of practical and scientific importance for disease control. In this study, the relative expression of TaLHY and TaPR-4 and TaPR-5 genes was higher in a resistant (Yinong 18) and moderately resistant (Pin 9928) cultivars rather than susceptible (Dongxuan 3) cultivar at 72 h post inoculation (hpi) with T. controversa. Similarly, the expression of defensin, TaPR-2 and TaPR-10 genes was observed higher in resistant and moderately resistant cultivars after exogenous application of phytohormones, including methyl jasmonate, salicylic acid, and abscisic acid. Laser confocal microscopy was used to track the fungal hyphae in the roots, leaves, and tapetum cells, which of susceptible cultivar were infected harshly by T. controversa than moderately resistant and resistant cultivars. There were no fungal hyphae in tapetum cells in susceptible cultivar after methyl jasmonate, salicylic acid and abscisic acid treatments. Moreover, after T. controversa infection, the pollen germination was of 80.06, 58.73, and 0.67% in resistant, moderately resistant and susceptible cultivars, respectively. The above results suggested that the use using of resistant cultivar is a good option against the dwarf bunt disease.
Keywords: TaPR genes; laser confocal microscopy; pollen grain; tapetum; wheat dwarf bunt.
Conflict of interest statement
All authors declare that there is no conflict of interest.
Figures






References
-
- Ghosh T., Pradhan C., Das A.B. Control of stem-rot disease of rice caused by Sclerotium oryzae catt and its cellular defense mechanism—A review. Physiol. Mol. Plant Pathol. 2020;112:101536. doi: 10.1016/j.pmpp.2020.101536. - DOI
-
- Trione E.J. Dwarf bunt of wheat and its importance in international wheat trade. Plant Dis. 1982;66:1083. doi: 10.1094/PD-66-1083. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

