Effect of Penetration Enhancers on Toenail Delivery of Efinaconazole from Hydroalcoholic Preparations
- PMID: 33809569
- PMCID: PMC8000921
- DOI: 10.3390/molecules26061650
Effect of Penetration Enhancers on Toenail Delivery of Efinaconazole from Hydroalcoholic Preparations
Abstract
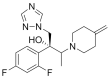
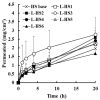
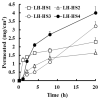
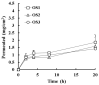
The incorporation of permeation enhancers in topical preparations has been recognized as a simple and valuable approach to improve the penetration of antifungal agents into toenails. In this study, to improve the toenail delivery of efinaconazole (EFN), a triazole derivative for onychomycosis treatment, topical solutions containing different penetration enhancers were designed, and the permeation profiles were evaluated using bovine hoof models. In an in vitro permeation study in a Franz diffusion cell, hydroalcoholic solutions (HSs) containing lipophilic enhancers, particularly prepared with propylene glycol dicaprylocaprate (Labrafac PG), had 41% higher penetration than the HS base. Moreover, the combination of hydroxypropyl-β-cyclodextrin with Labrafac PG further facilitated the penetration of EFN across the hoof membrane. In addition, this novel topical solution prepared with both lipophilic and hydrophilic enhancers was physicochemically stable, with no drug degradation under ambient conditions (25 °C, for 10 months). Therefore, this HS system can be a promising tool for enhancing the toenail permeability and therapeutic efficacy of EFN.
Keywords: bovine hoof; efinaconazole; hydroalcoholic solution; hydroxypropyl-β-cyclodextrin; onychomycosis; permeation enhancer; propylene glycol dicaprylocaprate; transungual drug delivery.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- de Berker D. Nails and hair. Medicine. 2009;37:286–290. doi: 10.1016/j.mpmed.2009.02.014. - DOI
-
- Siu W.J.J., Tatsumi Y., Senda H., Pillai R., Nakamura T., Sone D., Fothergill A. Comparison of In Vitro ntifungal activities of efinaconazole and currently available antifungal agents against a variety of pathogenic fungi associated with onychomycosis. Antimicrob. Agents Chemother. 2013;57:1610–1616. doi: 10.1128/AAC.02056-12. - DOI - PMC - PubMed
-
- Sugiura K., Sugimoto N., Hosaka S., Katafuchi-Nagashima M., Arakawa Y., Tatsumi Y., Siu W.J., Pillai R. The low keratin affinity of efinaconazole contributes to its nail penetration and fungicidal activity in topical onychomycosis treatment. Antimicrob. Agents Chemother. 2014;58:3837–3842. doi: 10.1128/AAC.00111-14. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- NRF-2019R1C1C1004211/This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning
- This research has been done by the author(s) working at the Department of Pharmacy of Dankook University. Department of Pharmacy was supported by the Research-Focused Department Promotion Project as a part of the University Innovation Support Program 2020
LinkOut - more resources
Full Text Sources
Other Literature Sources

