Immune-Stimulatory Effects of Curcumin on the Tumor Microenvironment in Head and Neck Squamous Cell Carcinoma
- PMID: 33809574
- PMCID: PMC8001767
- DOI: 10.3390/cancers13061335
Immune-Stimulatory Effects of Curcumin on the Tumor Microenvironment in Head and Neck Squamous Cell Carcinoma
Abstract
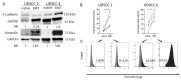
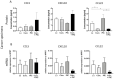
Curcumin is known to have immune-modulatory and antitumor effects by interacting with more than 30 different proteins. An important feature of curcumin is the inhibition of nuclear factor kappa of activated B-cells (NF-κB). Here, we evaluate the potential of curcumin to reverse the epithelial to mesenchymal transition (EMT) of head and neck squamous cell carcinoma (HNSCC) cells as a part of tumor escape mechanisms. We examined the impact of curcumin on the expression of different pro- and antitumoral chemokines in ex vivo HNSCC tumor tissue and primary macrophage cultures. Further, we evaluated the combinatorial effect of curcumin and toll-like receptor 3 (TLR3) agonist Poly I:C (PIC) on NF-κB inhibition and regulatory T-cell (Treg) attraction. Mesenchymal markers were significantly reduced in cancer specimens after incubation with curcumin, with simultaneous reduction of key transcription factors of EMT, Snail, and Twist. Furthermore, a decrease of the Treg-attracting chemokine CCL22 was observed. Additionally, curcumin-related inhibition of NF-κB nuclear translocation was evident. The combination of PIC with curcumin resulted in further NF-κB inhibition, whereas PIC alone contrarily resulted in NF-κB activation. Furthermore, curcumin was more effective in inhibiting PIC-dependent NF-κB activation and Treg attraction compared to known NF-κB inhibitors BAY 11-7082 or caffeic acid phenethyl ester (CAPE). The presented results show, for the first time, the immune-modulating effects of curcumin in HNSCC, with potent inhibition of the Treg-attracting effects of PIC. Hence, curcumin presents a promising drug in cancer therapy as a supplement to already established treatments.
Keywords: NF-κB; NF-κB inhibitors; Poly I:C; curcumin; epithelial to mesenchymal transition; head and neck squamous cell carcinoma; modulation of tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

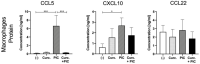


References
-
- Stewart B.W., Wild C.P. World Cancer Report 2014. International Agency for Research on Cancer; Lyon, France: 2014. IARC Publications Website-World Cancer Report 2014.
-
- Cooper J.S., Pajak T.F., Forastiere A.A., Jacobs J., Campbell B.H., Saxman S.B., Kish J.A., Kim H.E., Cmelak A.J., Rotman M., et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. - DOI - PubMed
-
- Ang K.K., Zhang Q., Rosenthal D.I., Nguyen-Tan P.F., Sherman E.J., Weber R.S., Galvin J.M., Bonner J.A., Harris J., El-Naggar A.K., et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin with or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J. Clin. Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

