Diet Transition from High-Forage to High-Concentrate Alters Rumen Bacterial Community Composition, Epithelial Transcriptomes and Ruminal Fermentation Parameters in Dairy Cows
- PMID: 33809588
- PMCID: PMC8002347
- DOI: 10.3390/ani11030838
Diet Transition from High-Forage to High-Concentrate Alters Rumen Bacterial Community Composition, Epithelial Transcriptomes and Ruminal Fermentation Parameters in Dairy Cows
Abstract
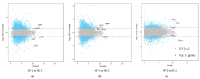
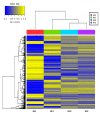
Effects of changing diet on rumen fermentation parameters, bacterial community composition, and transcriptome profiles were determined in three rumen-cannulated Holstein Friesian cows using a 3 × 4 cross-over design. Treatments include HF-1 (first high-forage diet), HC-1 (first high-concentrate diet), HC-2 (succeeding high-concentrate diet), and HF-2 (second high-forage diet as a recovery period). Animal diets contained Klein grass and concentrate at ratios of 8:2, 2:8, 2:8, and 8:2 (two weeks each), respectively. Ammonia-nitrogen and individual and total volatile fatty acid concentrations were increased significantly during HC-1 and HC-2. Rumen species richness significantly increased for HF-1 and HF-2. Bacteroidetes were dominant for all treatments, while phylum Firmicutes significantly increased during the HC period. Prevotella, Erysipelothrix, and Galbibacter significantly differed between HF and HC diet periods. Ruminococcus abundance was lower during HF feeding and tended to increase during successive HC feeding periods. Prevotellaruminicola was the predominant species for all diets. The RNA sequence analysis revealed the keratin gene as differentially expressed during the HF diet, while carbonic-anhydrase I and S100 calcium-binding protein were expressed in the HC diet. Most of these genes were highly expressed for HC-1 and HC-2. These results suggested that ruminal bacterial community composition, transcriptome profile, and rumen fermentation characteristics were altered by the diet transitions in dairy cows.
Keywords: bacterial community; changing diet; dairy cows; rumen fermentation; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Wetzels S., Mann E., Pourazad P., Qumar M., Pinior B., Metzler-Zebeli B., Wagner M., Schmitz-Esser S., Zebeli Q. Epimural bacterial community structure in the rumen of Holstein cows with different responses to a long-term subacute ruminal acidosis diet challenge. J. Dairy Sci. 2017;100:1829–1844. doi: 10.3168/jds.2016-11620. - DOI - PubMed
-
- Kleen J., Cannizzo C. Incidence, prevalence and impact of SARA in dairy herds. Anim. Feed Sci. Technol. 2012;172:4–8. doi: 10.1016/j.anifeedsci.2011.12.003. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

