If Virchow and Ehrlich Had Dreamt Together: What the Future Holds for KRAS-Mutant Lung Cancer
- PMID: 33809660
- PMCID: PMC8002337
- DOI: 10.3390/ijms22063025
If Virchow and Ehrlich Had Dreamt Together: What the Future Holds for KRAS-Mutant Lung Cancer
Abstract
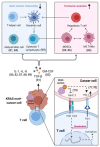
Non-small-cell lung cancer (NSCLC) with Kirsten rat sarcoma (KRAS) mutations has notoriously challenged oncologists and researchers for three notable reasons: (1) the historical assumption that KRAS is "undruggable", (2) the disease heterogeneity and (3) the shaping of the tumor microenvironment by KRAS downstream effector functions. Better insights into KRAS structural biochemistry allowed researchers to develop direct KRAS(G12C) inhibitors, which have shown early signs of clinical activity in NSCLC patients and have recently led to an FDA breakthrough designation for AMG-510. Following the approval of immune checkpoint inhibitors for PDL1-positive NSCLC, this could fuel yet another major paradigm shift in the treatment of advanced lung cancer. Here, we review advances in our understanding of the biology of direct KRAS inhibition and project future opportunities and challenges of dual KRAS and immune checkpoint inhibition. This strategy is supported by preclinical models which show that KRAS(G12C) inhibitors can turn some immunologically "cold" tumors into "hot" ones and therefore could benefit patients whose tumors harbor subtype-defining STK11/LKB1 co-mutations. Forty years after the discovery of KRAS as a transforming oncogene, we are on the verge of approval of the first KRAS-targeted drug combinations, thus therapeutically unifying Paul Ehrlich's century-old "magic bullet" vision with Rudolf Virchow's cancer inflammation theory.
Keywords: G12C inhibitor; ICI; KRAS; LKB1; NSCLC; immunotherapy; lung cancer; magic bullet.
Conflict of interest statement
J.K. received research support from Eli Lilly and Company and the German Cancer Aid Foundation. He previously served as a consultant and on advisory boards for Boehringer Ingelheim. P.A.J. reports consulting fees from AstraZeneca, Boehringer Ingelheim, Pfizer, Roche/Genentech, Takeda Oncology, ACEA Biosciences, Eli Lilly and Company, Araxes Pharma, Ignyta, Mirati Therapeutics, Novartis, Loxo Oncology, Daiichi Sankyo, Sanofi Oncology, Voronoi, SFJ Pharmaceuticals, Silicon Therapeutics, Transcenta, Syndax, Nuvalent, Bayer, Esai and Biocartis; receiving postmarketing royalties from DFCI-owned intellectual property on EGFR mutations licensed to Lab Corp; sponsored research agreements with AstraZeneca, Daichi-Sankyo, PUMA, Boehringer Ingelheim, Eli Lilly and Company, Revolution Medicines and Astellas Pharmaceuticals; and stock ownership in Loxo Oncology and Gatekeeper Pharmaceuticals.
Figures

References
-
- Barlesi F., Mazieres J., Merlio J.-P., Debieuvre D., Mosser J., Lena H., Ouafik L.H., Besse B., Rouquette I., Westeel V., et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. - DOI - PubMed
-
- Lusk C.M., Watza D., Dyson G., Craig D.B., Ratliff V., Wenzlaff A.S., Lonardo F., Bollig-Fischer A., Bepler G., Purrington K.S., et al. Profiling the Mutational Landscape in Known Driver Genes and Novel Genes in African American Non–Small Cell Lung Cancer Patients. Clin. Cancer Res. 2019;25:4300–4308. doi: 10.1158/1078-0432.CCR-18-2439. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

