Apoptotic Effects of Anthocyanins from Vitis coignetiae Pulliat Are Enhanced by Augmented Enhancer of the Rudimentary Homolog (ERH) in Human Gastric Carcinoma MKN28 Cells
- PMID: 33809701
- PMCID: PMC8002340
- DOI: 10.3390/ijms22063030
Apoptotic Effects of Anthocyanins from Vitis coignetiae Pulliat Are Enhanced by Augmented Enhancer of the Rudimentary Homolog (ERH) in Human Gastric Carcinoma MKN28 Cells
Abstract
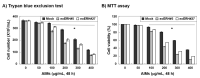
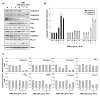
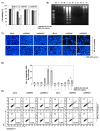
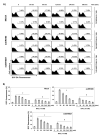
Evidence suggests that augmented expression of a certain gene can influence the efficacy of targeted and conventional chemotherapies. Here, we tested whether the high expression of enhancer of the rudimentary homolog (ERH), which serves as a prognostic factor in some cancers, can influence the efficacy of anthocyanins isolated from fruits of Vitis coignetiae Pulliat, Meoru in Korea (AIMs) on human gastric cancer cells. The anticancer efficacy of AIMs was augmented in ERH-transfected MKN28 cells (E-MKN28 cells). Molecularly, ERH augmented AIM-induced caspase-dependent apoptosis by activating caspase-3 and -9. The ERH-augmented apoptotic effect was related to mitochondrial depolarization and inhibition of antiapoptotic proteins, XIAP, and Bcl-2. In addition, reactive oxygen species (ROS) generation was augmented in AIMs-treated E-MKN28 cells compared to AIMs-treated naïve MKN28 cells. In conclusion, ERH augmented AIM-induced caspase-dependent mitochondrial-related apoptosis in MKN28 cells. A decrease in expression of Bcl-2 and subsequent excessive ROS generation would be the mechanism for ERH-augmented mitochondrial-related apoptosis in AIMs-treated MKN28 cells. A decrease in expression of XIAP would be another mechanism for ERH-augmented caspase-dependent apoptosis in AIMs-treated MKN28 cells.
Keywords: MKN28 human gastric carcinoma cells; Vitis coignetiae Pulliat; anthocyanins; anticancer effects; apoptosis; enhancer of the rudimentary homolog.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sasako M., Sakuramoto S., Katai H., Kinoshita T., Furukawa H., Yamaguchi T., Nashimoto A., Fujii M., Nakajima T., Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. - DOI - PubMed
-
- Noh S.H., Park S.R., Yang H.K., Chung H.C., Chung I.J., Kim S.W., Kim H.H., Choi J.H., Kim H.K., Yu W., et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi: 10.1016/S1470-2045(14)70473-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

