Ugi Reaction on α-Phosphorated Ketimines for the Synthesis of Tetrasubstituted α-Aminophosphonates and Their Applications as Antiproliferative Agents
- PMID: 33809715
- PMCID: PMC8002371
- DOI: 10.3390/molecules26061654
Ugi Reaction on α-Phosphorated Ketimines for the Synthesis of Tetrasubstituted α-Aminophosphonates and Their Applications as Antiproliferative Agents
Abstract
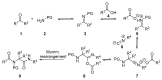
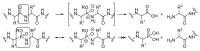
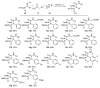
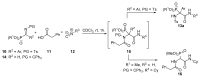
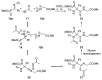
An Ugi three-component reaction using preformed α-phosphorated N-tosyl ketimines with different isocyanides in the presence of a carboxylic acid affords tetrasubstituted α-aminophosphonates. Due to the high steric hindrance, the expected acylated amines undergo a spontaneous elimination of the acyl group. The reaction is applicable to α-aryl ketimines bearing a number of substituents and several isocyanides. In addition, the densely substituted α-aminophosphonate substrates showed in vitro cytotoxicity, inhibiting the growth of carcinoma human tumor cell line A549 (carcinomic human alveolar basal epithelial cell).
Keywords: Ugi reaction; antiproliferative effect; multicomponent synthesis; tetrasubstituted carbons; α-aminophosphonates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
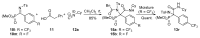
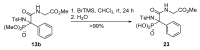
Similar articles
-
Synthesis of Tetrasubstituted Phosphorus Analogs of Aspartic Acid as Antiproliferative Agents.Molecules. 2022 Nov 18;27(22):8024. doi: 10.3390/molecules27228024. Molecules. 2022. PMID: 36432120 Free PMC article.
-
Asymmetric Synthesis of Functionalized Tetrasubstituted α-Aminophosphonates through Enantioselective Aza-Henry Reaction of Phosphorylated Ketimines.J Org Chem. 2015 Jan 2;80(1):156-64. doi: 10.1021/jo502233m. Epub 2014 Dec 9. J Org Chem. 2015. PMID: 25422859
-
Synthesis of novel antiproliferative hybrid bis-(3-indolyl)methane phosphonate derivatives.Eur J Med Chem. 2018 Oct 5;158:874-883. doi: 10.1016/j.ejmech.2018.09.011. Epub 2018 Sep 15. Eur J Med Chem. 2018. PMID: 30253344
-
The Catalytic Enantioselective Ugi Four-Component Reactions.Angew Chem Int Ed Engl. 2018 Dec 10;57(50):16266-16268. doi: 10.1002/anie.201811129. Epub 2018 Nov 15. Angew Chem Int Ed Engl. 2018. PMID: 30431219 Free PMC article. Review.
-
Asymmetric Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives.Molecules. 2021 May 27;26(11):3202. doi: 10.3390/molecules26113202. Molecules. 2021. PMID: 34071844 Free PMC article. Review.
Cited by
-
Hydroxy- and Amino-Phosphonates and -Bisphosphonates: Synthetic Methods and Their Biological Applications.Front Chem. 2022 Jun 1;10:890696. doi: 10.3389/fchem.2022.890696. eCollection 2022. Front Chem. 2022. PMID: 35721002 Free PMC article. Review.
-
Synthesis of Tetrasubstituted Phosphorus Analogs of Aspartic Acid as Antiproliferative Agents.Molecules. 2022 Nov 18;27(22):8024. doi: 10.3390/molecules27228024. Molecules. 2022. PMID: 36432120 Free PMC article.
-
Theoretical exploration on structures, bonding aspects and molecular docking of α-aminophosphonate ligated copper complexes against SARS-CoV-2 proteases.Front Pharmacol. 2022 Oct 3;13:982484. doi: 10.3389/fphar.2022.982484. eCollection 2022. Front Pharmacol. 2022. PMID: 36263127 Free PMC article.
-
Relationship between Structure and Antibacterial Activity of α-Aminophosphonate Derivatives Obtained via Lipase-Catalyzed Kabachnik-Fields Reaction.Materials (Basel). 2022 May 27;15(11):3846. doi: 10.3390/ma15113846. Materials (Basel). 2022. PMID: 35683150 Free PMC article.
References
-
- Knapp J.M., Kurth M.J., Shaw J.T., Younai A. In: Diversity-Oriented Synthesis. Trabocchi A., editor. Willey; New York, NY, USA: 2013. pp. 29–57.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

