Ugi Reaction on α-Phosphorated Ketimines for the Synthesis of Tetrasubstituted α-Aminophosphonates and Their Applications as Antiproliferative Agents
- PMID: 33809715
- PMCID: PMC8002371
- DOI: 10.3390/molecules26061654
Ugi Reaction on α-Phosphorated Ketimines for the Synthesis of Tetrasubstituted α-Aminophosphonates and Their Applications as Antiproliferative Agents
Abstract
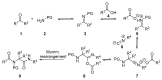
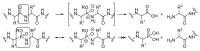
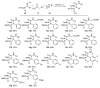
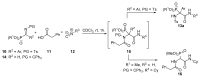
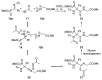
An Ugi three-component reaction using preformed α-phosphorated N-tosyl ketimines with different isocyanides in the presence of a carboxylic acid affords tetrasubstituted α-aminophosphonates. Due to the high steric hindrance, the expected acylated amines undergo a spontaneous elimination of the acyl group. The reaction is applicable to α-aryl ketimines bearing a number of substituents and several isocyanides. In addition, the densely substituted α-aminophosphonate substrates showed in vitro cytotoxicity, inhibiting the growth of carcinoma human tumor cell line A549 (carcinomic human alveolar basal epithelial cell).
Keywords: Ugi reaction; antiproliferative effect; multicomponent synthesis; tetrasubstituted carbons; α-aminophosphonates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
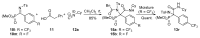
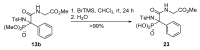
References
-
- Knapp J.M., Kurth M.J., Shaw J.T., Younai A. In: Diversity-Oriented Synthesis. Trabocchi A., editor. Willey; New York, NY, USA: 2013. pp. 29–57.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

