Evolution of a Multiple Sex-Chromosome System by Three-Sequential Translocations among Potential Sex-Chromosomes in the Taiwanese Frog Odorrana swinhoana
- PMID: 33809726
- PMCID: PMC8002213
- DOI: 10.3390/cells10030661
Evolution of a Multiple Sex-Chromosome System by Three-Sequential Translocations among Potential Sex-Chromosomes in the Taiwanese Frog Odorrana swinhoana
Abstract
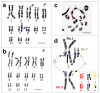
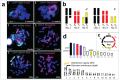
Translocation between sex-chromosomes and autosomes generates multiple sex-chromosome systems. It happens unexpectedly, and therefore, the evolutionary meaning is not clear. The current study shows a multiple sex chromosome system comprising three different chromosome pairs in a Taiwanese brown frog (Odorrana swinhoana). The male-specific three translocations created a system of six sex-chromosomes, ♂X1Y1X2Y2X3Y3-♀X1X1X2X2X3X3. It is unique in that the translocations occurred among three out of the six members of potential sex-determining chromosomes, which are known to be involved in sex-chromosome turnover in frogs, and the two out of three include orthologs of the sex-determining genes in mammals, birds and fishes. This rare case suggests sex-specific, nonrandom translocations and thus provides a new viewpoint for the evolutionary meaning of the multiple sex chromosome system.
Keywords: autosome; fusion; hexavalent; sex-chromosome turnover.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study.
Figures
Similar articles
-
Highly rapid and diverse sex chromosome evolution in the Odorrana frog species complex.Dev Growth Differ. 2022 Aug;64(6):279-289. doi: 10.1111/dgd.12800. Epub 2022 Aug 25. Dev Growth Differ. 2022. PMID: 35881001 Free PMC article.
-
Genomic Anatomy of Homozygous XX Females and YY Males Reveals Early Evolutionary Trajectory of Sex-determining Gene and Sex Chromosomes in Silurus Fishes.Mol Biol Evol. 2024 Aug 2;41(8):msae169. doi: 10.1093/molbev/msae169. Mol Biol Evol. 2024. PMID: 39136558 Free PMC article.
-
Meiotic behaviour of evolutionary sex-autosome translocations in Bovidae.Chromosome Res. 2016 Sep;24(3):325-38. doi: 10.1007/s10577-016-9524-x. Epub 2016 Apr 30. Chromosome Res. 2016. PMID: 27136937
-
Dynamics of vertebrate sex chromosome evolution: from equal size to giants and dwarfs.Chromosoma. 2016 Jun;125(3):553-71. doi: 10.1007/s00412-015-0569-y. Epub 2015 Dec 29. Chromosoma. 2016. PMID: 26715206 Review.
-
An evolutionary witness: the frog rana rugosa underwent change of heterogametic sex from XY male to ZW female.Sex Dev. 2007;1(6):323-31. doi: 10.1159/000111764. Epub 2008 Jan 18. Sex Dev. 2007. PMID: 18391544 Review.
Cited by
-
Understanding the genetic sex-determining mechanism in Hyla eximia treefrog inferred from H-Y antigen.PLoS One. 2024 May 31;19(5):e0304554. doi: 10.1371/journal.pone.0304554. eCollection 2024. PLoS One. 2024. PMID: 38820287 Free PMC article.
-
Evolution and regulation of animal sex chromosomes.Nat Rev Genet. 2025 Jan;26(1):59-74. doi: 10.1038/s41576-024-00757-3. Epub 2024 Jul 18. Nat Rev Genet. 2025. PMID: 39026082 Review.
-
The first linkage map for Australo-Papuan Treefrogs (family: Pelodryadidae) reveals the sex-determination system of the Green-eyed Treefrog (Litoria serrata).Heredity (Edinb). 2023 Oct;131(4):263-272. doi: 10.1038/s41437-023-00642-5. Epub 2023 Aug 4. Heredity (Edinb). 2023. PMID: 37542195 Free PMC article.
-
Chromosome-level echidna genome illuminates evolution of multiple sex chromosome system in monotremes.Gigascience. 2025 Jan 6;14:giae112. doi: 10.1093/gigascience/giae112. Gigascience. 2025. PMID: 39778707 Free PMC article.
-
Measurement of Chromosomal Arms and FISH Reveal Complex Genome Architecture and Standardized Karyotype of Model Fish, Genus Carassius.Cells. 2021 Sep 7;10(9):2343. doi: 10.3390/cells10092343. Cells. 2021. PMID: 34571992 Free PMC article.
References
-
- Smith D.A.S., Gordon I.J., Traut W., Herren J., Collins S., Martins D.J., Saitoti K., Ireri P., Ffrench-Constant R. A neo-W chromosome in a tropical butterfly links colour pattern, male-killing, and speciation. Proc. R. Soc. B Biol. Sci. 2016;283:20160821. doi: 10.1098/rspb.2016.0821. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

