Microbial Diversity and Community Variation in the Intestines of Layer Chickens
- PMID: 33809729
- PMCID: PMC8002243
- DOI: 10.3390/ani11030840
Microbial Diversity and Community Variation in the Intestines of Layer Chickens
Abstract
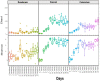
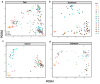
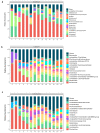
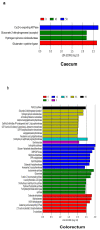
The intestinal microbiota is increasingly recognized as an important component of host health, metabolism and immunity. Early gut colonizers are pivotal in the establishment of microbial community structures affecting the health and growth performance of chickens. White Lohmann layer is a common commercial breed. Therefore, this breed was selected to study the pattern of changes of microbiota with age. In this study, the duodenum, caecum and colorectum contents of white Lohmann layer chickens from same environment control farm were collected and analyzed using 16S rRNA sequencing to explore the spatial and temporal variations in intestinal microbiota. The results showed that the diversity of the microbial community structure in the duodenum, caecum and colorectum increased with age and tended to be stable when the layer chickens reached 50 days of age and the distinct succession patterns of the intestinal microbiota between the duodenum and large intestine (caecum and colorectum). On day 0, the diversity of microbes in the duodenum was higher than that in the caecum and colorectum, but the compositions of intestinal microbes were relatively similar, with facultative anaerobic Proteobacteria as the main microbes. However, the relative abundance of facultative anaerobic bacteria (Escherichia) gradually decreased and was replaced by anaerobic bacteria (Bacteroides and Ruminococcaceae). By day 50, the structure of intestinal microbes had gradually become stable, and Lactobacillus was the dominant bacteria in the duodenum (41.1%). The compositions of dominant microbes in the caecum and colorectum were more complex, but there were certain similarities. Bacteroides, Odoribacter and Clostridiales vadin BB60 group were dominant. The results of this study provide evidence that time and spatial factors are important factors affecting the intestinal microbiota composition. This study provides new knowledge of the intestinal microbiota colonization pattern of layer chickens in early life to improve the intestinal health of layer chickens.
Keywords: 16S rRNA; diversity and community; intestinal microbiome; white Lohmann layer chickens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

