Prevention of Covid-19 Infection and Related Complications by Ozonized Oils
- PMID: 33809879
- PMCID: PMC8004285
- DOI: 10.3390/jpm11030226
Prevention of Covid-19 Infection and Related Complications by Ozonized Oils
Abstract
Background: The COVID-19 pandemic continues to ravage the human population; therefore, multiple prevention and intervention protocols are being rapidly developed. The aim of our study was to develop a new chemo-prophylactic/-therapeutic strategy that effectively prevents COVID-19 and related complications.
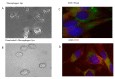
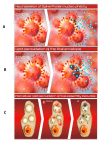
Methods: In in vitro studies, COVID-19 infection-sensitive cells were incubated with human oropharyngeal fluids containing high SARS-CoV-2 loads. Levels of infection were determined via intra-cellular virus loads using quantitative PCR (qPCR). Efficacies for infection prevention were determined using several antiviral treatments: lipid-encapsulated ozonized oil (HOO), water-soluble HOO (HOOws), UV, and hydrogen peroxide. In in vivo studies, safety and efficacy of HOO in fighting COVID-19 infection was evaluated in human subjects.
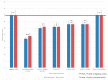
Results: HOO in combination with HOOws was the only treatment able to fully neutralize SARS-CoV-2 as well as its capacity to penetrate and reproduce inside sensitive cells. Accordingly, the feasibility of using HOO/HOOws was tested in vivo. Analysis of expired gas in healthy subjects indicates that HOO administration increases oxygen availability in the lung. For our human studies, HOO/HOOws was administered to 52 cancer patients and 21 healthy subjects at high risk for COVID-19 infection, and all of them showed clinical safety. None of them developed COVID-19 infection, although an incidence of at least 11 cases was expected. Efficacy of HOO/HOOws was tested in four COVID-19 patients obtaining recovery and qPCR negativization in less than 10 days.
Conclusions: Based on our experience, the HOO/HOOws treatment can be administered at standard doses (three pills per day) for chemo-prophylactic purposes to healthy subjects for COVID-19 prevention and at high doses (up to eight pills per day) for therapeutic purposes to infected patients. This combined prevention strategy can provide a novel protocol to fight the COVID-19 pandemic.
Keywords: COVID-19; COVID-19 challenge test; SARS-CoV-2; chemoprophylaxis; oxidative stress; prevention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Jiang H.J., Chen N., Shen Z.Q., Jing Y., Gang Q.Z., Jing M., Wei Y.Z., Yang S.D., Wang H.R., Wei W.X., et al. Inactivation of Poliovirus by Ozone and the Impact of Ozone on the Viral Genome. Biomed. Environ. Sci. 2019;32:324–333. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

