Bactericidal Properties of Rod-, Peanut-, and Star-Shaped Gold Nanoparticles Coated with Ceragenin CSA-131 against Multidrug-Resistant Bacterial Strains
- PMID: 33809901
- PMCID: PMC8004255
- DOI: 10.3390/pharmaceutics13030425
Bactericidal Properties of Rod-, Peanut-, and Star-Shaped Gold Nanoparticles Coated with Ceragenin CSA-131 against Multidrug-Resistant Bacterial Strains
Erratum in
-
Correction: Bucki et al. Bactericidal Properties of Rod-, Peanut-, and Star-Shaped Gold Nanoparticles Coated with Ceragenin CSA-131 against Multidrug-Resistant Bacterial Strains. Pharmaceutics 2021, 13, 425.Pharmaceutics. 2024 Oct 24;16(11):1354. doi: 10.3390/pharmaceutics16111354. Pharmaceutics. 2024. PMID: 39598609 Free PMC article.
Abstract
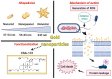
Background: The ever-growing number of infections caused by multidrug-resistant (MDR) bacterial strains requires an increased effort to develop new antibiotics. Herein, we demonstrate that a new class of gold nanoparticles (Au NPs), defined by shape and conjugated with ceragenin CSA-131 (cationic steroid antimicrobial), display strong bactericidal activity against intractable superbugs.
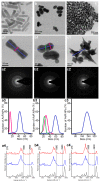
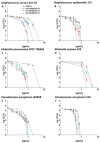
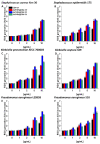
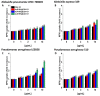
Methods: For the purpose of research, we developed nanosystems with rod- (AuR NPs@CSA-131), peanut-(AuP NPs@CSA-131) and star-shaped (AuS NPs@CSA-131) metal cores. Those nanosystems were evaluated against bacterial strains representing various groups of MDR (multidrug-resistant) Gram-positive (MRSA, MRSE, and MLSb) and Gram-negative (ESBL, AmpC, and CR) pathogens. Assessment of MICs (minimum inhibitory concentrations)/MBCs (minimum bactericidal concentrations) and killing assays were performed as a measure of their antibacterial activity. In addition to a comprehensive analysis of bacterial responses involving the generation of ROS (reactive oxygen species), plasma membrane permeabilization and depolarization, as well as the release of protein content, were performed to investigate the molecular mechanisms of action of the nanosystems. Finally, their hemocompatibility was assessed by a hemolysis assay.
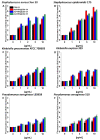
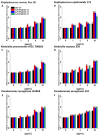
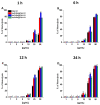
Results: All of the tested nanosystems exerted potent bactericidal activity in a manner resulting in the generation of ROS, followed by damage of the bacterial membranes and the leakage of intracellular content. Notably, the killing action occurred with all of the bacterial strains evaluated, including those known to be drug resistant, and at concentrations that did not impact the growth of host cells.
Conclusions: Conjugation of CSA-131 with Au NPs by covalent bond between the COOH group from MHDA and NH3 from CSA-131 potentiates the antimicrobial activity of this ceragenin if compared to its action alone. Results validate the development of AuR NPs@CSA-131, AuP NPs@CSA-131, and AuS NPs@CSA-131 as potential novel nanoantibiotics that might effectively eradicate MDR bacteria.
Keywords: Au NPs; CSA-131; MDR (multidrug-resistant); ceragenins; nanosystems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. [(accessed on 4 March 2021)]; Available online: https://www.who.int/news-room/detail/29-04-2019-new-report-calls-for-urg....
-
- Biggest Threats and Data, 2019 AR Threats Report. [(accessed on 27 July 2020)];2019 Available online: https://www.cdc.gov/drugresistance/biggest-threats.html.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

