An Overview on Anodes for Magnesium Batteries: Challenges towards a Promising Storage Solution for Renewables
- PMID: 33809914
- PMCID: PMC8004101
- DOI: 10.3390/nano11030810
An Overview on Anodes for Magnesium Batteries: Challenges towards a Promising Storage Solution for Renewables
Abstract
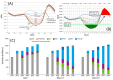
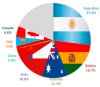
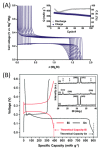
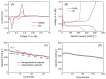
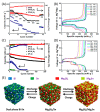
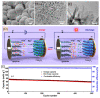
Magnesium-based batteries represent one of the successfully emerging electrochemical energy storage chemistries, mainly due to the high theoretical volumetric capacity of metallic magnesium (i.e., 3833 mAh cm-3 vs. 2046 mAh cm-3 for lithium), its low reduction potential (-2.37 V vs. SHE), abundance in the Earth's crust (104 times higher than that of lithium) and dendrite-free behaviour when used as an anode during cycling. However, Mg deposition and dissolution processes in polar organic electrolytes lead to the formation of a passivation film bearing an insulating effect towards Mg2+ ions. Several strategies to overcome this drawback have been recently proposed, keeping as a main goal that of reducing the formation of such passivation layers and improving the magnesium-related kinetics. This manuscript offers a literature analysis on this topic, starting with a rapid overview on magnesium batteries as a feasible strategy for storing electricity coming from renewables, and then addressing the most relevant outcomes in the field of anodic materials (i.e., metallic magnesium, bismuth-, titanium- and tin-based electrodes, biphasic alloys, nanostructured metal oxides, boron clusters, graphene-based electrodes, etc.).
Keywords: Mg metal; Sn-Bi alloy; anode; magnesium battery; post-Li battery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Guzmán A., Pinto-Gutiérrez C., Trujillo M.A. Attention to global warming and the success of envi-ronmental initial coin offerings: Empirical evidence. Sustainability. 2020;12:9885. doi: 10.3390/su12239885. - DOI
-
- Kim S., Lee J.E., Kim D. Searching for the Next New Energy in Energy Transition: Comparing the Impacts of Economic Incentives on Local Acceptance of Fossil Fuels, Renewable, and Nuclear Energies. Sustain. J. Rec. 2019;11:2037. doi: 10.3390/su11072037. - DOI
-
- Martins F., Felgueiras C., Smitkova M., Caetano N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies. 2019;12:964. doi: 10.3390/en12060964. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

