Effects of Bovine Colostrum with or without Egg on In Vitro Bacterial-Induced Intestinal Damage with Relevance for SIBO and Infectious Diarrhea
- PMID: 33809940
- PMCID: PMC8004259
- DOI: 10.3390/nu13031024
Effects of Bovine Colostrum with or without Egg on In Vitro Bacterial-Induced Intestinal Damage with Relevance for SIBO and Infectious Diarrhea
Abstract
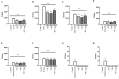
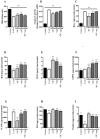
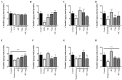
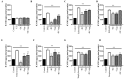
Small intestinal bacterial overgrowth (SIBO) occurs commonly, is difficult to treat, and frequently recurs. Bovine colostrum (BC) and chicken eggs contain immunoglobulins and other components that possess antimicrobial, immunoregulatory, and growth factor activities; however, it is not known if they have the ability to reduce injury caused by the presence of bacteria associated with SIBO (Streptococcus, Escherichia coli, Staphylococcus, Bacteroides, Klebsiella, Enterococcus, and Proteus) and infectious diarrhea (enteropathogenic Escherichia coli, Salmonella). We examined the effects of BC, egg, or the combination, on bacterial growth and bacteria-induced changes in transepithelial electrical resistance (TEER) and bacterial translocation across confluent Caco-2 monolayers. BC, egg, or the combination did not affect bacterial growth. Adding bacteria to monolayers reduced TEER and (with minor variations among species) increased bacterial translocation, increased monolayer apoptosis (increased caspase-3 and Baxα, reduced Bcl2), increased intercellular adhesion molecule 1 (ICAM-1), and reduced cell adhesion molecules zonulin1 (ZO1) and claudin-1. BC, egg, or the combination reduced these effects (all p < 0.01) and caused additional increases in vascular endothelial growth factor (VEGF) and Heat Shock Protein 70 (Hsp70) expression. We conclude that BC ± egg strengthens mucosal integrity against a battery of bacteria relevant for SIBO and for infectious diarrhea. Oral BC ± egg may have clinical value for these conditions, especially SIBO where eradication of precipitating organisms may be difficult to achieve.
Keywords: antimicrobial; irritable bowel syndrome; leaky gut; nutraceuticals; repair; small intestinal bacterial overgrowth (SIBO).
Conflict of interest statement
PanTheryx Inc. supply BC to the USA, European, and Asia-Pacific markets and are also the producers of DiaResQTM, a bovine colostrum and egg combination. Data included in this publication form part of the U.S. provisional patent application U.S. 62/978,104 in which R.J.P., N.C., and P.K. are named inventors. This does not affect our adherence to the
Figures

Similar articles
-
[Small intestinal bacterial overgrowth. An update].Rev Med Chil. 2005 Nov;133(11):1361-70. doi: 10.4067/s0034-98872005001100013. Epub 2005 Dec 29. Rev Med Chil. 2005. PMID: 16446861 Review. Spanish.
-
Small intestinal bacterial overgrowth in patients with irritable bowel syndrome.Gut. 2007 Jun;56(6):802-8. doi: 10.1136/gut.2006.108712. Epub 2006 Dec 5. Gut. 2007. PMID: 17148502 Free PMC article.
-
Duodenal and rectal mucosal microbiota related to small intestinal bacterial overgrowth in diarrhea-predominant irritable bowel syndrome.J Gastroenterol Hepatol. 2020 May;35(5):795-805. doi: 10.1111/jgh.14910. Epub 2019 Nov 5. J Gastroenterol Hepatol. 2020. PMID: 31674052
-
[Clinical features of irritable bowel syndrome with small intestinal bacterial overgrowth and a preliminary study of effectiveness of Rifaximin].Zhonghua Yi Xue Za Zhi. 2016 Jun 28;96(24):1896-902. doi: 10.3760/cma.j.issn.0376-2491.2016.24.005. Zhonghua Yi Xue Za Zhi. 2016. PMID: 27373356 Chinese.
-
Current views on the etiopathogenesis, clinical manifestation, diagnostics, treatment and correlation with other nosological entities of SIBO.Adv Med Sci. 2015 Mar;60(1):118-24. doi: 10.1016/j.advms.2014.09.001. Epub 2014 Oct 6. Adv Med Sci. 2015. PMID: 25657082 Review.
Cited by
-
The Use of Bovine Colostrum in Medical Practice and Human Health: Current Evidence and Areas Requiring Further Examination.Nutrients. 2021 Dec 27;14(1):92. doi: 10.3390/nu14010092. Nutrients. 2021. PMID: 35010967 Free PMC article.
-
IgYs: on her majesty's secret service.Front Immunol. 2023 Jun 12;14:1199427. doi: 10.3389/fimmu.2023.1199427. eCollection 2023. Front Immunol. 2023. PMID: 37377972 Free PMC article. Review.
-
Diverse Immune Effects of Bovine Colostrum and Benefits in Human Health and Disease.Nutrients. 2021 Oct 26;13(11):3798. doi: 10.3390/nu13113798. Nutrients. 2021. PMID: 34836054 Free PMC article. Review.
-
Colostrum and Lactoferrin Protect against Side Effects of Therapy with Antibiotics, Anti-inflammatory Drugs and Steroids, and Psychophysical Stress: A Comprehensive Review.Biomedicines. 2023 Mar 27;11(4):1015. doi: 10.3390/biomedicines11041015. Biomedicines. 2023. PMID: 37189633 Free PMC article. Review.
-
Effects of Chicken Egg Powder, Bovine Colostrum, and Combination Therapy for the Treatment of Gastrointestinal Disorders.Nutrients. 2024 Oct 29;16(21):3684. doi: 10.3390/nu16213684. Nutrients. 2024. PMID: 39519517 Free PMC article. Review.
References
-
- Middleton S.J., Playford R.J. Oxford Textbook of Medicine. 6th ed. Oxford University Press; Oxford, UK: 2020. Bacterial overgrowth of the small intestine; pp. 2879–2883.
-
- Mello C.S., Rodrigues M.S.D.C., Filho H.B.D.A., Melli L.C.F.L., Tahan S., Pignatari A.C.C., De Morais M.B. Fecal microbiota analysis of children with small intestinal bacterial overgrowth among residents of an urban slum in Brazil. J. Pediatr. 2018;94:483–490. doi: 10.1016/j.jped.2017.09.003. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

