(Pro)renin Receptor Is Present in Human Sperm and It Adversely Affects Sperm Fertility Ability
- PMID: 33809946
- PMCID: PMC8004193
- DOI: 10.3390/ijms22063215
(Pro)renin Receptor Is Present in Human Sperm and It Adversely Affects Sperm Fertility Ability
Abstract
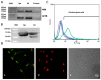
Sperm fertility ability may be modulated by different molecular systems, such as the renin-angiotensin system (RAS). Although renin is one of its most relevant peptides, the presence and role of the (pro)renin receptor (PRR) is completely unknown. We have proved for the first time the existence of PRR and its transcript in human sperm by western blot and RT-PCR. Immunofluorescence studies showed that this receptor is mainly located in the apical region over the acrosome and in the postacrosomal region of the sperm head and along the sperm tail. In addition, this prospective cohort study also proves that semen samples with higher percentages of PRR-positive spermatozoa are associated with poor sperm motility, worse blastocyst development and no-viable blastocysts. Our results provide insight into how PRR play a negative role in sperm physiology that it may condition human embryo quality and development. An in-depth understanding of the role of PRR in sperm fertility can help elucidate its role in male infertility, as well as establish biomarkers for the diagnosis or selection of sperm to use during assisted reproductive techniques.
Keywords: assisted; embryonic development; human; prorenin receptor; reproductive techniques; semen analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Larson-Cook K.L., Brannian J.D., Hansen K.A., Kasperson K.M., Aamold E.T., Evenson D.P. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil. Steril. 2003;80:895–902. doi: 10.1016/S0015-0282(03)01116-6. - DOI - PubMed
-
- Meseguer M., Pellicer A., Garrido N., Martínez-Conejero J.A., Simón C., Remohí J. Relationship Between Standard Semen Parameters, Calcium, Cholesterol Contents, and Mitochondrial Activity in Ejaculated Spermatozoa From Fertile and Infertile Males. J. Assist. Reprod. Genet. 2004;21:445–451. doi: 10.1007/s10815-004-8761-7. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

